Abstract
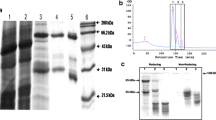
Splenocytes, derived from mice that had been immunized with protoplasts prepared from suspension cultures of root cells of Glycine max (L.) Merr. (SB-1 cell line), were fused with a murine myeloma cell line. The resulting hybridoma cultures were screened for the production of antibodies directed against the soybean protoplasts and were then cloned. One monoclonal antibody, designated MVS-1, was found to bind to the outer surface of the plasma membrane on the basis of several criteria: (a) agglutination of the protoplasts; (b) binding of fluorescence-labeled immunoglobulin on protoplasts yielding a ring staining pattern with prominent intensity at the edges; and (c) saturable binding by protoplasts of 125I-labeled Antibody MVS-1. The antigenic target of Antibody MVS-1, identified by immunoblotting techniques, contained a polypeptide of relative molecular mass (Mr) approx. 400000 under both reducing and non-reducing conditions. When the antigenic target of Antibody MVS-1 was chromatographed in potassium phosphate buffer, the position of elution corresponded to that of a high-molecular-weight species (Mr 400000). These results provide the protein characterization required for the analysis of the mobility of Antibody MVS-1 bound to the plasma membrane of SB-1 cells.
Similar content being viewed by others
Abbreviations
- D:
-
diffusion coefficient
- Mr :
-
relative molecular mass
- PBS:
-
phosphate-buffered saline (8.00 g NaCl, 1.15 g Na2HPO4, 0.20 g NaH2PO4 per 1 L, pH 7.2)
- TPBS:
-
phosphate-buffered saline containing 0.5% Tween-20
- TX-100, TX-114:
-
Triton X-100, X-114
- SDS:
-
sodium dodecyl sulfate
References
Beiss, U. (1963) Phosphatides and glycolipids. In: Modern methods of plant analysis, vol. 6, pp. 56–58, Linskens, H.F., Tracey, M.V., eds. Springer-Verlag, Berlin Göttingen Heidelberg
Blake, M.S, Johnston, K.H., Russell-Jones, G.J., Gotschlich, E.C. (1984) A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on western blots. Anal. Biochem. 136, 175–179
Bordier, C. (1981) Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 256, 1604–1607
Burgess, J., Linstead, P.J., Fisher, V.E.L. (1977) Studies on higher plant protoplasts by scanning electron microscopy. Micron 8, 113–122
Chamberlain, J.P. (1979) Fluorographic detection of radioactivity in polyacrylamide gels with the water soluble fluor, sodium salicylate. Anal. Biochem. 98, 132–135
Crittenden, S.L., Roff, C.F., Wang, J.L. (1984) Carbohydrate binding protein 35. Identification of the galactose specific lectin in various tissues of the mouse. Mol. Cell. Biol. 4, 1252–1259
Fraker, P.J., Speck, J.C. (1978) Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1, 3, 4, 6 tetrachloro — 3a, 6a-diphenylglycoluril. Biochem. Biophys. Res. Commun. 80, 849–857
Galfre, G., Milstein, C. (1981) Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 73, 1–46
Hancock, K., Tsang, V.C.W. (1983) India ink stain of proteins on nitrocellulose paper. Anal. Biochem. 133, 157–162
Heide, K., Schwick, G. (1978) Salt fractionation of immunoglobulins. In: Handbook of experimental immunology, vol. 1, pp. 1–7.11 Weir, D.M. ed. Blackwell, London
Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685
Metcalf, T.N., III, Szabo, L.J., Schubert, K.R., Wang, J.L. (1980) Immunochemical identification of an actin-like protein from soybean seedlings. Nature 285, 171–172
Metcalf, T.N., III, Szabo, L.J., Schubert, K.R., Wang, J.L. (1984) Ultrastructural and immunochemical analysis of the distribution of microfilaments in seedlings and plants of Glycine max. Protoplasma 120, 91–99
Metcalf, T.N., III, Villanueva, M.A., Schindler, M., Wang, J.L. (1986) Monoclonal antibodies directed against protoplasts of soybean cells. Analysis of the lateral mobility of surface bound Antibody MVS-1. J. Cell Biol. 102, 1350–1357
Metcalf, T.N., III, Wang, J.L., Schubert, K.R., Schindler, M. (1983) Lectin receptors on the plasma membrane of soybean cells. Binding and lateral diffusion of lections Biochemistry 22, 3969–3975
Mishell, B.B., Shiigi, S.M. (eds) (1980) Selected methods in cellular immunology. W. H. Freeman, San Francisco
Nagata, T., Takebe, I. (1970) Cell wall regeneration and cell division in isolated tobacco mesophyll protoplasts. Planta 92, 301–308
Peters, R. (1981) Translational diffusion in the plasma membrane of single cells by fluorescence microphotolysis. Cell Biol. Int. Rep. 5, 733–759
Ramirez-Salcedo, J., Salcedo-Hernandez, R., Celis, H. (1983) Extraction of detergents from hydrophobic proteins with isopentanol: Application to electrophoretic analysis of photosynthetic bacterial hydrophobic proteins. Anal. Biochem. 132, 324–327
Scarborough, G.A. (1975) Isolation and characterization of Neurospora crassa plasma membranes. J. Biol. Chem. 250, 1106–1111
Scatchard, G. (1949) The attraction of proteins for small molecules and ions. Ann. N.Y. Acad. Sci. 51, 660–672
Schibeci, A., Fincher, G.B., Stone, B.A., Wardrop, A.B. (1982) Isolation of plasma membrane from protoplasts of Lolium multiforum (ryegrass) endosperm cells. Biochem. J. 205, 511–519
Sela, B.-A., Wang, J.L., Edelman, G.M. (1975) Isolation of lectins of different specificities on a single affinity adsorbent. J. Biol. Chem. 250, 7535–7538
Taylor, J.A., West, D.W. (1980) The use of Evan's blue stain to test the survival of plant cells after exposure to high salt and high osmotic pressure. J. Exp. Bot. 31, 571–576
Towbin, H., Staehelin, T., Gordon, G. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 76, 4350–4354
Wang, J.L., Metcalf, T.N., III, Schindler, M. (1983) Lateral diffusion of lectin receptors on the plasma membrane of soybean cells. In: Chemical taxonomy, molecular biology, and function of plant lectins, pp. 273–276. Goldstein, I.J., Etzler, M.E., eds., Alan R. Liss, New York
Williamson, F.A., Fowke, L.C. Constabel, F.C., Gamborg, O.L. (1976) Labeling of concanavalin A sites on the plasma membrane of soybean protoplasts. Protoplasma 89, 305–316
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Villanueva, M.A., Metcalf, T.N. & Wang, J.L. Monoclonal antibodies directed against protoplasts of soybean cells. Planta 168, 503–511 (1986). https://doi.org/10.1007/BF00392269
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00392269