Summary
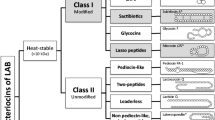
A large number of new bacteriocins in lactic acid bacteria (LAB) has been characterized in recent years. Most of the new bacteriocins belong to the class II bacteriocins which are small (30–100 amino acids) heat-stable and commonly not post-translationally modified. While most bacteriocin producers synthesize only one bacteriocin, it has been shown that several LAB produce multiple bacteriocins (2–3 bacteriocins).
Based on common features, some of the class II bacteriocins can be divided into separate groups such as the pediocin-like and strong anti-listeria bacteriocins, the two-peptide bacteriocins, and bacteriocins with a sec-dependent signal sequence. With the exception of the very few bacteriocins containing a sec-dependent signal sequence, class II bacteriocins are synthesized in a preform containing an N-terminal double-glycine leader. The double-glycine leader-containing bacteriocins are processed concomitant with externalization by a dedicated ABC-transporter which has been shown to possess an N-terminal proteolytic domain. The production of some class II bacteriocins (plantaricins of Lactobacillus plantarum C11 and sakacin P of Lactobacillus sake) have been shown to be transcriptionally regulated through a signal transduction system which consists of three components: an induction factor (IF), histidine protein kinase (HK) and a response regulator (RR). An identical regulatory system is probably regulating the transcription of the sakacin A and carnobacteriocin B2 operons. The regulation of bacteriocin production is unique, since the IF is a bacteriocin-like peptide with a double-glycine leader processed and externalized most probably by the dedicated ABC-transporter associated with the bacteriocin. However, IF is not constituting the bacteriocin activity of the bacterium, IF is only activating the transcripion of the regulated class II bacteriocin gene(s).
The present review discusses recent findings concerning biosynthesis, genetics, and regulation of class II bacteriocins.
Similar content being viewed by others
References
Abee T 1995. Pore-forming bacteriocins of Gram-positive bacteria and self-protection mechanisms of producer organisms. FEMS Microbiol. Lett. 129: 1–10
Allison GE, C Fremaux and TR Klaenhammer 1994. Expansion of bacteriocin activity and host range upon complementation of two peptides encoded within the Lactacin F operon. J. Bacteriol. 176: 2235–2241
Allison GE, RW Worobo, ME Stiles and TR Klaenhammer 1995. Heterologous expresion of the lactacin F peptides by Carnobacterium piscicola LV17. Appl. Environ. Microbiol. 61: 1371–1377
Aymerich T, H Holo, LS Håvarstein, M Hugas, M Garriga, IF Nes 1996. Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl. Environ. Microbiol. 62: 1676–1682
Axelsson L, A Holck, SE Birkeland, T Aukrust and H Blom 1993. Cloning and nucleotide sequencing of a gene from Lactobacillus sake LB706 necessary for sakacin A production and immunity. Appl. Environ. Microbiol. 59: 2868–2875
Axelsson L and A Holck 1995. The genes involved in production of and immunity to sakacin A, a bacteriocin from Lactobacillus sake Lb706. J. Bacteriol. 177: 2125–2137
Balaban N and RP Novick 1995. Autocrine regulation of toxin synthesis by Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 92: 1619–1623
Bourret RB, KA Borkovich and MI Simon 1991. Signal transduction pathways involving protein phosphorylation in prokaryotes. An. Rev. Biochem. 60: 401–441
Brurberg MB, IF Nes and V Eijsink 1996. Unpublished results
Chikindas MJ, MJ Garcia Garcera, AMDriessen, AM Lederboer, J Nissen-Meyer, IF Nes, T Abee, WN Konings and G Venema 1993. Pediocin PA-1, a bacteriocin from Pediococcus acidilactici PAC1.0, forms hydrophillic pores in the cytoplasmatic membrane of target cells. Appl. Environ. Microbiol. 59: 3577–3584
Chikindas ML, K Venema, AM Ledeboer, G Venema and J Kok 1995. Expression of lactococcin A and pediocin PA-1 in heterologous hosts. Letters in Applied Microbiol. 21: 183–189
De Vos WM, OP Kuipers, JRvan der Meer and RJ Siezen 1995. Maturation pathway of nisin and other lantibiotics: post-translationally modified antimicrobial peptides exported by gram-positive bacteria. Mol. Microbiol. 17: 427–337
Diep DB, LS Håvarstein, J Nissen-Meyer and IF Nes 1994. The gene encoding plantaricin, a bacteriocin from Lactobacillus plantarum C11, is located on the same transcription unit as an agr-like regulatory system. Appl. Environ. Microbiol. 60: 160–166
Diep DB, LS Håvarstein and IF Nes 1995. A bacteriocin-like peptide induces bacteriocin synthesis in L. plantarum C11. Mol. Microbiol. 18: 631–639
Diep DB, 1996. Characterization of the locus responsible for the bacteriocin production in Lactobacillus plantarum C11. J. Bacteriol. 178: 4472–4483
Eisjink VGH, MB Brurberg, PH Middelhoven and IF Nes 1996. Induction of bacteriocin production in Lactobacillus sake by a secreted peptide. J. Bacteriol. 178: 2232–2237
Engelke G, Z Gutowski-Eckel, P Kiesau, K Siegers, M Hammelmann and KD Entien 1994. Regulation of nisin biosynthesis and immunity of Lactococcus lactic 6F3. Appl. Environ. Microbiol. 60: 814–825
Fath MJ, LH Zhang, J Rush and R Kolter 1994. Purification and characterization of colicin V from Eschericia coli culture supernatants. Biochemistry 33: 6911–6917
Fimland G, OR Blingso, K Sletten, G Jung, IF Nes, J Nissen-Meyer 1996. New biological activity hybrid bacteriocins constructed by combining regions from various pediocin-like bacteriocins. Appl. Environ. Microbiol. 62: 000–000
Franke CM, KJ Leenhout, AJ Haandrikman, J Kok, G Venema and K Venema 1996. Topology of LcnD, a protein implicated in the transport of bacteriocins from Lactococcus lactic. J. Bacteriol. 176: 1766–1769
Fremaux C, C Ahn and TR Klaenhammer 1993. Molecular analysis of the lactocin F operon. Appl. Environ. Microbiol. 59: 3906–3915
Gilmore MS, RA Segarra, MC Booth, CP Bogie, LR Hall and DB Clewell 1994. Genetic structure of the Enterococcus faecalis plasmid pAD1-encoded cytolytic system and its relationship to lantibiotic determinants. J. Bacteriol. 176: 7335–7344
Gilson L, HK Mahanty, R Kolter (1990). Genetic analysis of an MDR-like export system: the secretion of colicin V. EMBO J. 9: 3875–3884
Guangyong J, RC Blavis and RP Novick (1995). Cell density of staphylococcal virulence mediated by an octapeptide pheromone. Proc. Natl. Acad. Sci. USA 92: 12055–12059
Hastings JW, M Sailer, K Johnson, KL Roy, JC Vederas and ME Stiles 1991. Characterization of leucocin A-UAL 187 and cloning of the bacteriocin gene from Leuconostoc gelidum. J. Bacteriol. 173: 7491–7500
Hechard Y, DB Derijard, F Letellier and Y Cenatiempo 1992. Characterization and purification of mesentericin Y105, an anti-Listeria bacteriocin from Leuconostoc mesenteroides. J. Gen. Microbiol. 138: 2725–2731
Henderson JT, AL Chopko and PDvan Wasserman 1992. Purification and primary structure of pediocin PA-1 produced by Pediococcus acidilactici PAC1.0. Arch. Biochem. Biophys. 295: 5–12
Hoch JA and TJ Silhavy (Eds.), 1995. Two-Component Signal Transduction. ASM Press, Washington, D.C. USA
Holck A, L Axelsson, SE Birkeland, T Aukrust and H Blom 1992. Purification and amino acid sequence of sakacin A, a bacteriocin from Lactobacillus sake Lb 706. J. Gen. Microbiol. 138: 2715–2720
Holo H. (1996) Resistance and immunity to bacteriocins of lactic acid bacteria. Manuscript submitted to Can. J. Microbiol.
Holo H, Ø Nilssen and IF Nes 1991. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J. Bacteriol. 173: 3879–3887
Huehne K, A Holck, L Axelson and L Kroeckel 1996. Analysis of sakacin P gene cluster from Lactobacillus sake LB674 and its expression in sakacin P negative L. sake strains. Microbiology. 142: 1437–1448
Håvarstein LS, H Holo and IF Nes 1994. The leader peptide of colicin V shares consensus sequences with leader peptides that are common amongst peptide bacteriocins produced by Grampositive bacteria. Microbiol. 140: 2383–2389
Håvarstein LS, G Coomaraswamy and DA Morrison 1995. An unidentified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 92: 11140–11144
Håvarstein LS, BD Diep and IF Nes 1995. A Family of bacteriocin ABC transporters carry out proteolytic processing of their substrates concomitant with export. Mol. Microbiol. 16: 229–240
Jack RW, JR Tagg and B Ray (1995). Bacteriocins of Gram-Positive Bacteria. Microbiol. Rev. 59: 171–200
Jiménez-Diaz R, JL Ruiz-Barba, DP Cathcart, H Holo, IF Nes, KH Sletten and PJ Warner 1995. Purification and partial amino acid sequence of plantaricin S, a bacteriocin produced by Lactobacillus plantarum LPCO10, the activity of which depends on the complementary action of two peptides. Appl. Environ. Microbiol. 61: 4459–4463
Jiménez-Diaz R, RM Rios-Sánchez, M Desmazeaud, JL Ruiz-Barba and JC Piard 1993. PlantaricinS and T, two new bactriocins produced by Lactobacillus plantarum LPCO10 isolated from a green olive fermentation. Appl. Environ. Microbiol. 59: 1416–1424
Kanatani K, M Oshimura and K Sano 1995. Isolation and charcterization of acidocin A and cloning of the bacteriocin gene from Lactobacillus acidophilus. Appl. Environ. Microbiol. 61: 1061–1067
Karlson P & M. Lüscher 1959. Pheromones a new term of class of biologically active substances? 183: 55–56
Klaenhammer TR 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12: 39–86
Kuipers OP, HS Rollema, WMG Yap, HJ Boot, RJ Siezen and WM deVos 1992. Engineering dehydrated amino acid residues in the antimicrobial peptide nisin. J. Biol. Chem. 267: 24340–24346
Kuipers OP, MM Beerthuyzen, PGGA deRuyter, EJ Luesink and WM deVos 1995. Autoregulation of nisin biosynthesis in Lactococcus lactic by signal transduction. J. Biol. Chem. 270: 281–291
Konings RNH and CW Hilbers (Eds.), 1996. Lantibiotics: A Unique Group of Antibiotic Peptides. Antonie van Leeuwenhoek Int. J. Gen. Molec. Microbiol. 69: Issue 2
Larsen AG and B Nørrung 1993. Inhibition of Listeria monocytogenes by bavaricin A, a bacteriocin produced by Lactobacillus bavaricus MI401. Lett. Appl. Microbiol. 17: 132–134
Leer RL, JMBM van der Vossen, Mvan Giezen, JMvan Noort and PH Pouwels (1995): Genetic analysis of acidocin B, a novel bacteriocin produced by Lactobacillus acidophilus. Microbiol. 141: 1629–1635
Magnusson R, J Solomon and AD Grossman 1994. Biochemical and genetic characterization of a competence pheromone from B. subtilis. Cell 77: 207–216
Marugg JD, CF Gonzales, BS Kunka, AM Ledeboer, MJ Pucci, MY Toonen, SA Walker, LCM Zoetmulder and PA Vandenbergh 1992. Cloning, expression, and nucleotide sequence of genes involved in production of pediocin PA-1, a bacteriocin from Pediococcus acidilactici PAC1.0. Appl. Environ. Microbiol. 58: 2360–2367
Morfeldt E, L Janzon, S Arvidson and S Löfdalh 1988. Cloning of a chromosomal locus (exp) which regulates the expression of several exoprotein genes in Staphylococcus aureus. Mol. Gen. Genet. 211: 435–440
Motlagh AM, AK Buhunia, F Szostek, TR Hansen, MC Johnson, B Ray 1992. Nucleotide and amino acid sequence of pap gene pediocin AcH) produced in Pediococcus acididlactici H. Lett. Appl. Microbiol. 15: 45–48
Muriana PM and TR Klaenhammer 1991. Cloning, phenotypic expression, and DNA sequence of the gene for lactacin F, an antimicrobial peptide produced by Lactobacillus spp. J. Bacteriol. 173: 1779–1788
Nes IF, LS Håvarstein and H Holo 1995. Genetic of non-lantibiotics bacteriocins. In Genetics of Streptococci, Enterococci and Lactococci. (J. J. Ferretti, M. S. Gilmore, T. R. Klaenhammer, and F. Brown, Eds.). Karger, New York, pp. 645–651
Nieto Lozano JC, J Nissen-Meyer, K Sletten, C Peláz and IF Nes 1992. Purification and amino acid sequence of a bacteriocin produced by Pediococcus acidilactici. J. Gen. Microbiol. 138: 1985–1990
Nissen-Meyer J, H Holo, LS Håvarstein, K Sletten and IF Nes 1992. A novel lactococcal bacteriocin whose activity depends on the complementary action of two peptides. J. Bacteriol. 174: 5686–5692
Nissen-Meyer J, LS Håvarstein, H Holo, K Sletten and IF Nes 1993a. Association of the lactococcin A immunity factor with the cell membrane: purification and characterization of the immunity factor. J. Gen. Microbiol. 139: 1503–1509
Nissen-Meyer J, AG Larsen, K Sletten, M Daeschel and IF Nes 1993b. Purifiaction and characterization of plantaricin A, a Lactobacillus plantarum bacteriocin whose activity depends on the action of two peptides. J. Gen. Microbiol. 139: 1973–1978
O'Brian GJ and HK Mahanty 1996. Complete nucleotide sequence of microcin 24 genetic region and analysis of a new ABC transporter. Accession number ECU47048
Parkinson JS and EC Kofoid 1992. Communication modules in bacterial signaling proteins. Annu. Rev. Genet. 26: 71–112
Pestova EV, LS Håvarstein and DA Morrison 1996. Regulation of transformability by an auto-induced peptide pheromone and a two-component regulatory system. Molec. Microbiol. 21: 855–864
Piard JC, OP Kuipers, HS Rolema, MJ Deamzeaud and WM de Vos 1993. Structure, organization and expression of the lct gene for lacticin 481, a novel lantibiotic produced by Lactococcus lactic. J. Biol. Chem. 268: 16361–16368
Quadri LEN, KL Roy, JC Vederas and ME Stiles 1995a. Characterization of four genes involved in the production of antimicrobial peptides by Carnobacterium piscicola LV17B. Published DNA sequence with accession nr. 147121. em-bl.new
Quadri LEN, M Sailer, MR Terebiznik, KL Roy, JC Vederas and ME Stiles 1995b. Characterization of the protein conferring immunity to the antimicrobial peptide carnobacteriocin B2 and expression of the carnobacteriocins B2 and BM1. J. Bacteriol. 177: 1144–1151
Quadri LEN, M Sailers, KL Roy, JC Vederas and ME Stiles 1994. Chemical and genetic characterization of bacteriocins produced by Carnobacterium piscicola LV17B. J. Biol. Chem. 269: 12204–12211
Ray B 1992. Pediocin(s) of Pediococcus acidilactici as a Food Biopreservative. Page 265–322. In: Food Biopreservatives of Microbial Origin. Eds. B. Ray and M. Daeschel. CRC Press Inc. Bocan Raton, Forida
Ross KF, CW Ronson and JR Tagg 1993. Isolation and characterization of the lantibiotic salavaricin A and its structural gene salA from Streptococcus salivarius 20P3. Appl. Environ. Microbiol. 60: 1652–1657
Sahl HG, RW Jack and G Bierbaum (1995): Biosynthesis and biological activities of lantibiotics with unique post-translational modifications. Eur. J. Biochem. 230: 827–853
Saucier L, A Poon and ME Stiles (1995). Induction of bacteriocin in Carnobacterium piscicola LV 17. J. Appl. Bacteriol. 78: 684–690
Stock JB, AJ Ninfa and AM Stock 1989. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol. Rev. 53: 450–490
Stoddard GW, JP Petzel, MJ Van Belkum, J Kok and LL McKay 1992. Molecular analyses of the lactococcin A gene cluster from Lactococcus lactis subsp. lactis biovar diacetylactis WM4. Appl. Environ. Microbiol. 58: 1952–1961
Tichaczek PS, J Nissen-Meyer, IF Nes, RF Vogel and WP Hammes 1992. Characterization of the bacteriocins curvacin A from Lactobacillus curvatus LTH1174 and sakacin from L. sake LTH673. Syst. Appl. Microbiol. 15: 460–468
, RF Vogel and WP Hammes 1993. Cloning and sequencing of curA encoding curvacin A, the bacteriocin produced by Lactobacillus curvatus LTH1174. Arch. Microbiol. 160: 279–283
Tichaczek PS 1994. Cloning and sequencing of sakP encoding sakacin P, the bacteriocin produced by Lactobacillus sake LTH 673. Microbiology 140: 361–367
Van Belkum MJ, Hayema BJ, Leeninga RE, Kok J and G Venema (1991). Organization and nucleotide sequences of two lactococcal bacteriocin operons. Applied and Environmental Microbiology 57: 492–498
Van Belkum MJ, J Kok and G Venema 1992. Cloning, sequencing, and expression in Escherichia coli of lcnB, a third bacteriocin determinant from the lactococcal bacteriocin plasmid p9B4-6. Appl. Environ. Microbiol. 58: 572–577
Van Belkum MJ and ME Stiles (1995) Molecular characterization of genes involved in the production of the bacteriocin leucocin A from Leuconostoc gelidum. Appl. Environ. Microbiol. 61: 3573–3579
Van der Mer JR, J Polman, MM Beerthuyzen, RJ Siezen, O Kuipers and WM de Vos 1993. Characterization of the Lactococcus lactis nisin A operon genes nisP, encoding a subtilisin-like seine protease involved in precursor processing, and nisR, encoding a regulatory protein involved in nisin biosynthesis. J. Bacteriol. 175: 2578–2588
Venema K, T Abee, AJ Haandrikman, KJ Leenhouts, J Kok, WN Konings and GVenema 1993. Mode of action of lactococcin B, a thiol-activated bacteriocin from Lactococcus lactis. Appl. Environ. Microbiol. 59: 1041–1048
Venema K, RE Haverkort, T Abee, AJ Haandrikman, KJ Leenhouts, LD Leij, G Venema and J Kok 1994. Mode of action of LciA, the lactococcin A immunity protein. Mol. Microbiol. 16: 521–532
Venema K, J Kok, JD Marugg, MY Toonen, AM Ledeboer, G Venema and ML Chikindas 1995a. Functional analysis of the pediocin operon of Pediococcus acidilactici PAC1.0:PedB is the immunity protein and PedD is the precursor processing enzyme. Mol. Microbiol. 17: 515–522
Venema K, MHA Dost, G Venema and J Kok 1996. Mutational analysis and chemical modification of lactococcin B, a bacteriocin produced by Lactococcus lactis. Manuscript
Venema K, MHA Dost, PAH Beun, AJ Haandrikman, G Venema and J Kok 1996. The genes for secretion of lactococins are located on the chromosom of Lactococcus lactis IL1403. Appl. Environ. Microbiol. 62: 1689–1692
Worobo RW, T Henkel, M Sailer, KL Roy, JC Vederas and ME Stiles 1994. Characteristics and genetic determinant of a hydrophobic peptide bacteriocin, carnobacteriocin A, produced by Carnobacterium piscicola LV17A. Microbiology 140: 517–526
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nes, I.F., Diep, D.B., Håvarstein, L.S. et al. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie van Leeuwenhoek 70, 113–128 (1996). https://doi.org/10.1007/BF00395929
Issue Date:
DOI: https://doi.org/10.1007/BF00395929