Abstract
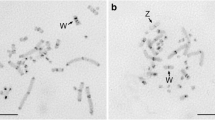
DNA from Plethodon cinereus cinereus separates into two fractions on centrifugation to equilibrium in neutral CsCl. The smaller of these fractions has been described as a high-density satellite. It represents about 2% of nuclear DNA from this species, and it has a density of 1.728 g/cm3. It is cytologically localized near the centromeres of all 14 chromosomes of the haploid set. In P. c. cinereus the heavy satellite DNA constitutes about 1/4 of the DNA in centromeric heterochromatin. The nature of the rest of the DNA in centromeric heterochromatin is unknown. The number of heavy satellite sequences clustered around the centromeres in a chromosome from P. c. cinereus is roughly proportional to the size of the chromosome, as determined by in situ hybridization with satellite-complementary RNA, and autoradiography. Likewise the amount of contromeric heterochromatin, as identified by its differential stainability with Giemsa, shows a clear relationship to chromosome size. — The heavy satellite sequences identified in DNA from P. c. cinereus are also present in smaller amounts in other closely related forms of Plethodon. Plethodon cinereus polycentratus and P. richmondi have approximately half as many of these sequences per haploid genome as P. c. cinereus. P. hoffmani and P. nettingi shenandoah have about 1/3 as many of these sequences as P. c. cinereus. P. c. cinereus, P. c. polycentratus, and P. richmondii all have detectable heavy satellites with densities of 1.728 g/cm3. Among these forms, satellite size as determined by optical density measurements, and number of satellite sequences as determined from hybridization studies, vary co-ordinately. P. c. cinereus heavy satellite sequences are not detectable in P. nettingi, P. n. hubrichti, or P. dorsalis. The latter species has a heavy satellite with a density of 1.718 g/cm3, representing about 8% of the genomic DNA, and two light satellites whose properties have not been investigated. The heavy satellite of P. dorsalis is cytologically localized in the centromeric heterochromatin of this species. — These observations are discussed in relation to the function and evolution of highly repetitive DNA sequences in the centromeric heterochromatin of salamanders and other organisms.
Similar content being viewed by others
References
Beattie, W. G., Skinner, D. M.: The diversity of satellite DNAs of Crustacea. Biochim. biophys. Aota (Amst.) 281, 169–178 (1972).
Bostock, C. J., Prescott, D. M., Hatch, F. T.: Timing of replication of the satellite and main band DNAs in cells of the kangeroo rat (Dipodomys ordii). Exp. Cell Res. 74, 487–495 (1972).
Britten, R. J., Davidson, E. H.: Repetitive and non-repetitive DNA sequences and a speculation on the origins of evolutionary novelty. Quart. Rev. Biol. 46, 111–138 (1971)
Britten, R. J., Kohne, D. E.: Repeated sequences in DNA. Science 161, 529–540 (1968)
Eckhardt, R. A., Gall, J. G.: Satellite DNA associated with heterochromatin in Rhynchosciara. Chromosoma (Berl.) 32, 407–427 (1971)
Flamm, W. G.: Highly repetitive sequences of DNA in chromosomes. Int. Rev. Cytol. 32, 1–51 (1972)
Flamm, W. G., Bond, H. E., Burr, H. E.: Density gradient centrifugation of DNA in a fixed angle rotor. Biochim. biophys. Acta (Amst.) 129, 310–317 (1966)
Gall, J. G., Cohen, E. H., Polan, M. L.: Repetitive DNA sequences in Drosophila. Chromosoma (Berl.) 33, 319–344 (1971)
Gall, J. G., Macgregor, H. C., Kidston, M. E.: Gene amplification in the oocytes of dytiscid water beetles. Chromosoma (Berl.) 26, 169–187 (1969)
Gall, J. G., Pardue, M. L.: Nucleic acid hybridization in cytological preparations. In: Methods in enzymology, vol. 21, part D, p. 470–480. New York-London: Academic Press 1971
Gallagher, A., Hewitt, G., Gibson, I.: Differential Giemsa staining of heterochromatic B-chromosomes in Myrmeleotettix maculatus (Thunb.) (Orthopter: Acrididae). Chromosoma (Berl.) 40, 167–172 (1973)
Hatch, F. T., Mazrimas, J. A.: Satellite DNAs in the kangaroo rat. Biochim. biophys. Acta (Amst.) 224, 291–294 (1970)
Hennig, W., Walker, P. M. B.: Variations in the DNA from two rodent families (Cricetidae and Muridae). Nature (Lond.) 225, 915–919 (1970)
Highton, R.: Distributional interactions among eastern North American salamanders of the genus Plethodon. Research Division Monograph 4, p. 139–187. Blacksburg, Va.: Virginia Polytechnic Institute and State University 1972.
Highton, R., Worthington, R. D.: A new salamander of the genus Plethodon from Virginia. Copeia (Wash.) 3, 617–626 (1967)
Jones, K. W.: Chromosomal and nuclear location of mouse satellite DNA in individual cells. Nature (Lond.) 225, 912–915 (1970)
Kit, S.: Equilibrium sedimentation in density gradients of DNA preparations from animal tissues. J. molec. Biol. 3, 711–716 (1961)
Laird, C. D., McConaughy, B. L., McCarthy, B. J.: Rate of fixation of nucleotide substitutions in evolution. Nature (Lond.) 224, 149–154 (1969)
Macgregor, H. C., Kezer, J.: The chromosomal localization of a heavy satellite DNA in the testis of Phethodon c. cinereus. Chromosoma (Berl.) 33, 167–182 (1971)
Mazrimas, J. A., Hatch, F. T.: Intranuclear distribution of satellite DNA from the kangaroo rat. Exp. Cell Res. 63, 462–466 (1970)
Mazrimas, J. A., Hatch, F. T.: A possible relationship between satellite DNA and the evolution of kangaroo rat species (genus Dipodomys). Nature (Lond.) New Biol. 240, 102–105 (1972)
Pardue, M. L., Gall, J. G.: Chromosomal localization of mouse satellite DNA. Science 168, 1356–1358 (1970)
Patau, K.: Absorption microphotometry of irregular shaped objects. Chromosoma (Berl.) 5, 341–362 (1952)
Southern, E. M.: Base sequence and evolution of Guinea-pig satellite DNA. Nature (Lond.) 227, 794–798 (1970)
Sumner, A. T.: A simple technique for demonstrating centromeric heterochromatin. Exp. Cell Res. 75, 304–306 (1972)
Swift, H.: Cytochemical techniques for nucleic acids. In: The nucleic acids (E. Chargaff and J. N. Davidson, eds.) vol. II, p. 51–92. New York: Academic Press 1955
Thurow, G. R.: On the small black Plethodon problem. Series in the Biological Sciences, No 6, 1–48. Western Illinois University 1968
Wake, D. B.: Comparative osteology and evolution of the lungless salamanders, family Plethodontidae. Mem. S. Calif. Acad. Sci. 4, 1–111 (1966)
Waring, M., Britten, R. J.: Nucleotide sequence repetition: a rapidly reassociating fraction of mouse DNA. Science 154, 791–794 (1966)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Macgregor, H.C., Horner, H., Owen, C.A. et al. Observations on centromeric heterochromatin and satellite DNA in salamanders of the genus Plethodon . Chromosoma 43, 329–348 (1973). https://doi.org/10.1007/BF00406742
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00406742