Summary
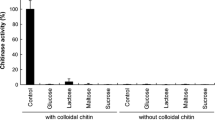
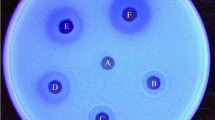
Decomposition of chitin by Cytophaga johnsonii was investigated. Unlike other chitinolytic bacteria, some strains of C. johnsonii did not liberate chitinase extracellularly; instead the cells of such strains had need for close contact with the chitin particles in order to hydrolyze them. The cell-free extract of one of these strains, viz., C. johnsonii C35 did show presence of chitinase. The remaining strains liberated an extracellular chitinase and a chitobiase. The partially purified chitinase from the culture filtrates of C. johnsonii C31 was most active at pH 6.3–6.5 and at 40°C.
Metabolism of N-acetylglucosamine, a product of chitin hydrolysis, by C. johnsonii C35 and C31 was investigated. The nature of some of the products formed and the steps involved in their transformations by the resting cells and the cell-free extracts suggested that N-acetylglucosamine first gets deacetylated to glucosamine before it is further oxidized to glucosaminic acid by these strains. Both strains were also able to dehydrogenate gluconate to 2-ketogluconate, the former being in all probability the product of deamination of glucosaminic acid.
Similar content being viewed by others

References
Anderson, R. L., Ordal, E. J.: Cytophaga succinicans sp. n., a facultatively anaerobic aquatic myxobacterium. J. Bact. 81, 130–138 (1961).
Berger, L. R., Reynolds, D. M.: The chitinase system of a strain of Streptomyces griseus. Biochim. biophys. Acta (Amst.) 29, 522–534 (1968).
Datta, A. G., Katznelson, H.: Oxidation of 2,5-diketogluconate by a cell-free enzymes preparation from Acetobacter melanogenum. Nature (Lond.) 179, 153–154 (1957).
Jeuniaux, C.: Purification of a streptomyces chitinase. Biochem. J. 66, 29 p. (1957).
—: Recherches sur les chitinases. II. Purification de la chitinase d'un streptomycete, et séparation électrophorétique de principes chitinolytiques, distincts. Arch. intern. Physiol. Biochim. 67, 597–617 (1969).
Kulka, D., Walker, T. K.: The ketogenic activities of Acetobacter species in a glucose medium. Arch. Biochem. 50, 169–179 (1954).
Kvamme, E., Hellman, L.: Isolation of pyruvic and α-Ketoglutaric acid from blood and tissues in the presence of carbon-14 acetate. Analyt. Chem. 26, 1995–1997 (1954).
Levvy, G. A., Conchie, J.: Mammalian glycosidase and their inhibition by aldonolactones. In: Methods in Enzymology, Vol. VIII, pp. 571–584, ed. by E. F. Neufeld and V. Ginsburg. New York: Academic Press 1966.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., Randall, R. J.: Protein measurement with the folin phenol reagent. J. biol. Chem. 193, 265–275 (1951).
Narrod, S. A., Wood, W. A.: Carbohydrate oxidation by Pseudomonas fluorescens. V. Evidence for gluconokinase and 2-ketogluconokinase. J. biol. Chem. 220, 45–55 (1956).
Partridge, S. M.: Filter-paper partition chromatography of sugars. 1. General description and application to the qualitative analysis of sugars in apple juices, egg white and foetal blood of sheep. Biochem. J. 42, 238–249 (1948).
—: Aniline hydrogen phthalate as sparying reagent for chromatography of sugars. Nature (Lond.) 164, 443 (1949).
Payne, W. J., Kieber, R. J.: The chromatographic determination of glucosamine with ninhydrin. Arch. Biochem. 52, 1–4 (1954).
Peterson, E. A., Sober, H. A.: In: Methods in enzymology, vol. V, pp. 3–27, ed. by S. P. Colowick and N. O. Kaplan. New York: Academic Press 1962.
Stanier, R. Y.: The cytophaga groups: A contribution to the biology of myxobacteria. Bact. Rev. 6, 143–196 (1942).
—: Studies on nonfruiting myxobacteria I. Cytophaga johnsonae, N. sp., A chitin decomposing myxobacterium. J. Bact. 53, 297–315 (1947).
Tracey, M. V.: Chitin. In: Modern methods of plant analysis, vol. 2, pp. 264–274, ed. by K. Paech and M. V. Tracey. Berlin-Göttingen-Heidelberg: Springer 1955.
Veldkamp, H.: A study of the aerobic decomposition of chitin by micro-organisms. Mededel. Landbouwhogesch. Wageningen 55, 127–174 (1955).
Wieland, Th., Fischer, E.: Papierchromatographie von α-Ketosäuren. Naturwissenschaften 36, 219 (1949).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sundarraj, N., Bhat, J.V. Breakdown of chitin by Cytophaga johnsonii . Archiv. Mikrobiol. 85, 159–167 (1972). https://doi.org/10.1007/BF00409298
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00409298