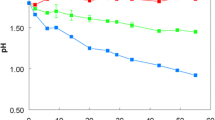
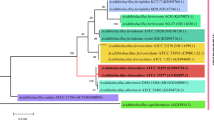
Abstract
The physiological properties of an organism isolated from a selective chemostat enrichment using acetate and thiosulphate as the limiting substrates, provisionally called Thiobacillus Q, were investigated. Although the organism made up 85% of the community in the enrichment culture, its expected chemolithotrophic nature was not apparent in batch experiments. The growth yield was not enhanced by the addition of thiosulphate to an acetate containing mineral medium, even though up to 50% of the thiosulphate was oxidized. Under acetate limitation in the chemostat, there was a linear increase in yield with thiosulphate addition up to a concentration of 7 mM. Higher thiosulphate concentrations resulted in loss of thiosulphate oxidizing capacity and a decrease in the biomass to the level obtained with acetate alone. This loss may be due to the presence of inhibitory (50–100 μM) levels of sulphite which is probably produced as an intermediate of the biological thiosulphate oxidation. Experiments with sulphide showed that Thiobacillus Q could also use it as an additional energy source. The complete lack of autotrophic growth, both in batch and chemostat experiments, together with the absence of even very low amounts of the key enzymes of the Calvin cycle demonstrated that this organism is a typical chemolithoheterotroph. Although this organism has provisionally been placed in the genus Thiobacillus, standard taxonomic procedures showed a close relationship with Pseudomonas alcaligenes. This study stresses the importance of quantitative chemostat studies in establishing the role of inorganic oxidations in energy metabolism and in the understanding of the role of heterotrophic sulphur oxidation in natural environments.
Similar content being viewed by others
References
Ames BN, Dubin DT (1960) The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem 235:769–775
Beudeker RF, Cannon GC, Kuenen JG, Shively JM (1980) Relations between D-ribulose-1,5-bisphosphate carboxylase, carboxysomes and CO2-fixing capacity in the obligate chemolithotroph Thiobacillus neapolitanus grown under different limitations in the chemostat. Arch Microbiol 124:185–189
Beudeker RF, Codd GA, Kuenen JG (1981) Quantification and intracellular distribution of ribulose-1,5-bisphosphate carboxylase in Thiobacillus neapolitanus, as related to possible functions of carboxysomes. Arch Microbiol 129:361–367
Beudeker RF, Gottschal JC, Kuenen JG (1982) Reactivity versus flexibility in thiobacilli. Antonie van Leeuwenhoek J Microbiol Serol 48:39–51
Bruinenberg PM, Dijken JP, v, Scheffers WA (1983) An enzymic analysis of NADPH production and consumption in Candida utilis. J Gen Microbiol 129:965–971
Chen PS, Toribara TY, Warner H, (1956) Microdetermination of phosphorous. Anal Chem 28:1756–1758
Fox GE, Stackebrandt E, Hespell RB, Gibson J, Manilof J, Dyer TA, Wolfe RS, Balch WE, Tanner RS, Magrum LJ, Zablen LB, Lakemore R, Gupta R, Bonen L, Lewis BJ, Stahl DA, Luehrsen KR, Chen KN, Woese CR (1980) The phylogeny of prokaryotes. Science 209:457–463
Friedrich CG, Mitrenga G (1981) Oxidation of thiosulphate by Paracoccus denitrificans and other hydrogen bacteria. FEMS Microbiol Lett 10:209–212
Goa J (1953) A microbiuret method for protein determination; determination of total protein in cerebrospinal fluid. J Clin Lab Invest 5:218–222
Gottschal JC, Kuenen JG (1980a) Selective enrichment of facultatively chemolithotrophic thiobacilli and related organisms in continuous culture. FEMS Microbiol Lett 7:241–247
Gottschal JC, Kuenen JG (1980b) Mixotrophic growth of Thiobacillus A2 on acetate and thiosulphate as growth limiting substrates in the chemostat. Arch Microbiol 126:33–42
Guittoneau G, Keilling J (1932) L'évolution et la solubilisation du soufre élémentaire dans la terre arable. Ann Agron NS 2:690–725
Harder W, Visser K, Kuenen JG (1974) Laboratory fermentor with an improved magnetic drive. Lab Pract 23:241–249
Harrison AP (1983) Genomic and physiological comparisons between heterotrophic Thiobacilli and Acidiphilum cryptum, Thiobacillus versutus sp. nov. and Thiobacillus acidophilus nom. rev. Int J Syst Bacteriol 33:211–217
Holthuijzen YA, Breemen JFL v, Kuenen JG, Konings WN (1986) Protein composition of the carboxysome of Thiobacillus neapolitanus. Arch Microbiol 144:398–404
Kämpf C, Pfennig N (1980) Capacity of Chromatiaceae for chemotrophic growth. Specific respiration rates of Thiocystis violacea and Chromatium vinosum. Arch Microbiol 127:125–135
Katayama-Fujimura Y, Kuraishi H (1983) Emendation of Thiobacillus perometabolis London and Rittenberg. Int J Syst Bacteriol 33:650–651
Kelly DP, Kuenen JG (1984) Ecology of the colourless sulphur bacteria. In: Codd GA (ed) Aspects of microbial metabolism and ecology. Academic Press, London, pp 211–239
Kelly DP, Harrison AP (1988) Genus Thiobacillus. In: Staley JT, Pfennig N, Holt JG (eds) Bergey's manual of systematic bacteriology, vol 3, 9th edn. Williams & Wilkinson, Baltimore (in press)
Kuenen JG (1988) Colorless sulfur bacteria In: Staley JT, Pfennig N, Holt JG (eds) Bergey's manual of systematic bacteriology, vol 3, 9th edn. Williams & Wilkinson, Baltimore (in press)
Kuenen JG, Beudeker RF (1982) Microbiology of thiobacilli and other sulphur-oxidizing autotrophs, mixotrophs and heterotrophs. Phil Trans R Soc Lond B 298:473–497
Lane DJ, Stahl DA, Olsen GJ, Heller DJ, Pace NR (1985) Phylogenetic analyses of the genera Thiobacillus and Thiomicrospira by 5S rRNA sequences. J Bacteriol 163:75–81
Leefeldt RH, Matin A (1980) Growth and physiology of Thiobacillus novellus under nutrient-limited mixotroph conditions. J Bacteriol 142:645–650
London J, Rittenberg SC (1967) Thiobacillus perometabolis nov. sp., a non-autotrophic Thiobacillus. Arch Mikrobiol 59:218–225
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Matin A (1978) Organic nutrition of chemolithotrophic bacteria. Ann Rev Microbiol 32:433–468
Matin A, Kaha FJ, Leefeldt RH (1980) Growth factor requirement of Thiobacillus novellus. Arch Microbiol 124:91–95
Mizoguchi T, Sato T, Okabe T (1976) New sulphur-oxidising bacteria capable of growing heterotrophically, Thiobacillus rubellus nov. sp. and Thiobacillus delicatus nov. sp. J. Ferment Techn 54:181–191
Palleroni NJ (1984) Section 4, Family 1: Pseudomonaceae. In: Krieg NR, Holt JG (eds) Bergey's manual of systematic bacteriology, vol 1, 9th edn. Williams & Wilkinson, Baltimore, London, pp 141–219
Perez RC, Matin A (1980) Growth of Thiobacillus novellus on mixed substrates (mixotrophic growth) J Bacteriol 142:633–638
Romano AH, Vranken NJ v., Preisand P, Brustolon M (1975) Regulation of the Thiobacillus intermedius glucose uptake system by thiosulfate. J Bacteriol 121:577–582
Rittenberg SC (1969) The role of exogenous organic matter in the physiology of chemolithotrophic bacteria. In: Rose AH, Williamson JF (eds) Advances in microbial physiology, vol 3. Academic Press, London, pp 159–196
Robertson LA, Kuenen JG (1983) Anaerobic and aerobic denitrification by sulphide oxidizing bacteria from waste water. Proc Europ Symp Anaerobic Waste Water Treatment, TNO, Noordwijkerhout, pp 3–12
Ruby EG, Wirsen CO, Jannasch HW (1981) Chemolithotrophic sulfur-oxidising bacteria from the Galanagos rift hydrothermal vents. Appl Environm Microbiol 42:317–324
Schie BJ v, Eijken JP v, Kuenen JG (1984) Non coordinated synthesis of glucose dehydrogenase and its prosthetic group PQQ in Acinetobacter and Pseudomonas species. FEMS Microbiol Lett 4:133–138
Schlegel HG, Kaltwasser H, Gottschalk G (1961) Ein Submersverfahren zur Kultur wasserstoffoxydierender Bakterien: Wachstumsphysiologische Untersuchungen. Arch Mikrobiol 38:209–222
Schook LB, Berk RS (1978) Nutritional studies with Pseudomonas aeruginosa grown on inorganic sulfur sources. J Bacteriol 133:1377–1382
Siebert K, Schobert P, Bowien B (1981) Purification, some catalytic and molecular properties of phosphoribulokinase from Alcaligenes eutrophus. Biochim Biophys Acta 658:35–44
Sijderius R (1946) Heterotrophe bacteriën, die thiosulfaat oxydeeren. Ph. D. Thesis Univ. Amsterdam
Skiba U, Wainwright M (1984) Oxidation of elemental-S in coastal dune sands and soils. Plant Soil 77:87–95
Sörbo B (1957) A colorimetric method for the determination of thiosulphate. Biochim Biophys Acta 23:412–416
Starkey RL (1935) Isolation of some bacteria which oxidize thiosulfate. Soil Science 39:197–219
Suylen GMH, Stefess GC, Kuenen JG (1986) Chemolithotrophic potential of a Hyphomicrobium species, capable of growth on methylated sulphur compounds. Arch Microbiol 146:192–198
Swaby RJ, Vitolins MI (1969) Sulphur oxidation in Australian soils. Trans Int Conf Soil Sci 4:673–681
Trüper HG, Schlegel HG (1964) Sulphur metabolism in Thiorhodaceae. 1. Quantitative measurements on growing cells of Chromatium okenii. Antonie van Leeuwenhoek J Microbiol Serol 30:225–238
Tuttle JH (1980) Organic carbon utilization by resting cells of thiosulfate-oxidising marine heterotrophs. Appl Environm Microbiol 40:516–521
Tutle JH, Jannasch HW (1972) Occurrence and types of Thiobacillus-like bacteria in the sea. Limnol Oceanogr 7:532–543
Tuttle JH, Jannasch HW (1973) Sulfide and thiosulfate-oxidising bacteria in anoxic marine basins. Marine Biology 20:64–70
Tuttle JH, Jannasch HW (1977) Thiosufate stimulation of microbial dark assimilation of carbon dioxide in shallow marine water. Microb Ecol 4:9–25
Tuttle JH, Holmes PE, Jannasch HW (1974) Growth rate stimulation of marine pseudomonads by thiosulfate. Arch Microbiol 99:1–14
Vishniac W, Santer M (1957) The thiobacilli. Bacteriol Rev 21:195–213
Vitolins MI, Swaby RJ (1969) Activity of sulphur-oxidising microorganisms in some Australian soils. Austr J Soil Res 7:171–183
Wainwright M (1984) Sulfur oxidation in soils. Adv in Agronomy 37:349–396
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gommers, P.J.F., Kuenen, J.G. Thiobacillus strain Q, a chemolithoheterotrophic sulphur bacterium. Arch. Microbiol. 150, 117–125 (1988). https://doi.org/10.1007/BF00425150
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00425150