Summary
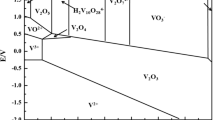
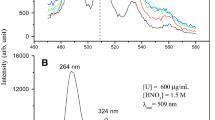
Salicylhydroxamic acid has been used as a colorimetric reagent for the estimation of uranium, vanadium, molybdenum and iron. It permits the direct estimation of vanadium in presence of molybdenum and uranium, though iron interferes, while the estimation of uranium or molybdenum is not possible in presence of each other or of vanadium. The vanadium complex can be removed from solution by extraction with ethyl acetate and estimated colorimetrically between pH 0.8 to 3.5. This permits its determination in steels after removal of iron. Sensitivity: U 0.1 μg, V 0.017 μg, Mo 0.015 μg, Fe 0.0125 μg.
Similar content being viewed by others
References
Bhaduri, A. S.: Z. analyt. Chem. 151, 109 (1956).
Das Gupta, A. K., and J. Gopta: J. Sci. Ind. Res. 9 B, 237 (1950).
Singh, M. M., and A. K. Das Gupta: J. Sci. Ind. Res. 8 B, 186 (1949).
Wise, W. M., and W. W. Brandt: Analyt. Chemistry 27, 1392 (1955); cf. Z. analyt. Chem. 152, 359 (1956).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bhaduri, A.S., Rây, P. Salicylhydroxamic acid as an analytical reagent. Z. Anal. Chem. 154, 103–113 (1956). https://doi.org/10.1007/BF00458381
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00458381