Abstract
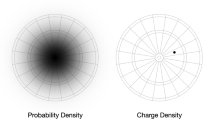
It is shown that the electronic charge density of a ground-state atom decreases monotonically as a function of radial distance from the nucleus, contrary to the widespread belief that the shell structure is reflected by relative maxima in the density. Any proposed relationship between chemical bonding and the maxima in the radial density functions of atoms should therefore be regarded with caution. It is proven that the electrostatic potential of an atom must be monotonically decreasing. The changes in charge distribution upon molecule formation are also discussed.
Similar content being viewed by others
References
Eyring,H., Walter,J., Kimball,G.E.: Quantum chemistry, pp. 166–167. New York: John Wiley & Sons 1944; Moeller,T.: Inorganic chemistry, p. 129. New York: John Wiley & Sons 1952; Atkins,P.W.: Molecular quantum mechanics, p. 261. Oxford: Clarendon Press 1970; Levine,I.N.: Quantum chemistry, Vol. I, p. 287. Boston: Allyn and Bacon 1970
Goodisman,J.: Theoret. Chim. Acta (Berl.)31, 101 (1973)
Dementi,E.: Tables of atomic wave functions. San Jose, Calif.: International Business Machines Corp. 1965
Cohen,M., Dalgarno,A.: Proc. Phys. Soc.77, 748 (1961); Kern,C.W., Karplus,M.: J. Chem. Phys.40, 1374 (1964)
Sperber,G.: Intern. J. Quantum Chem.5, 189 (1971)
Weeks,J.D., Hazi,A., Rice,S.A.: Advan. Chem. Phys.16, 283 (1969); Simons,G.: J. Chem. Phys.55, 756 (1971)
Slater,J.C.: Phys. Rev.36, 57 (1930); Slater,J.C.: J. Chem. Phys.41, 3199 (1964)
Clementi,E., Raimondi,D.L., Reinhardt,W.P.: J. Chem. Phys.47, 1300 (1967)
Mulliken,R.S.: J. Am. Chem. Soc.72, 4493 (1950)
See, for example: Bader,R.F.W., Henneker,W.H., Cade,P.E.: J. Chem. Phys.46, 3341 (1967); Ransil,B.J., Sinai,J.J.: J. Chem. Phys.46, 4050 (1967); Hazelrigg,Jr.,M.J., Politzer,P.: J. Phys. Chem.73, 1008 (1969)
Politzer,P.: Theoret. Chim. Acta (Berl.)16, 120 (1970)
See, for instance: Boyd,D.B.: J. Chem. Phys.52, 4846 (1970); Absar, I., Van Wazer, J. R.: J. Chem. Phys.56, 1284 (1972)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Weinstein, H., Politzer, P. & Srebrenik, S. A misconception concerning the electronic density distribution of an atom. Theoret. Chim. Acta 38, 159–163 (1975). https://doi.org/10.1007/BF00581473
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00581473