Abstract
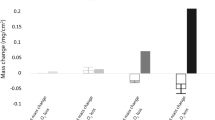
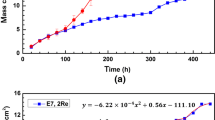
When Ni-20 Cr alloys and stainless steels are oxidized while submerged in molten salt (NaCl or NaCl-KCl), readily oxidizable alloy components, such as chromium, and in some cases iron, migrate to the surface to form non-adherent, granular, and thus nonprotective oxides. This loss of alloy constituents causes a counter current flow of vacancies which condense into an interconnecting pore network filled with salt. Since the salt penetrates into the structure, the loss of alloying metals does not require intermetallic diffusion over long distances, but instead corrosion products are largely removed by solution of alloying metals as ions in the pore-salt network. Evidence is presented which suggests that an integral part of the corrosion process is recrystallization, occurring in the porous zone, and that in the Ni-20 Cr alloy chromium diffuses down grain boundaries of the grain network to be deposited at grain boundary-pore intersections as chromium ions. The concentration gradient of chromium ions in the salt phase forces chromium diffusion out to the bulk salt where the higher effective oxygen pressure forms Cr2O3 and some chromate ion.
Similar content being viewed by others
References
P. Hancock,Corrosion of Alloys at High Temperatures in Atmospheres Consisting of Fuel Combustion Products and Associated Impurities (Her Majesty's Stationery Office, London, 1968).
E. J. Bradbury, P. Hancock, and H. Lewis,Metallurgia 67, 3 (1963).
W. D. Manly, J. H. Coobs, J. H. De Van, D. A. Douglas, H. Inouye, P. Patriarco, T. K. Roche and J. L. Scott, Second United Nations Conference.Peaceful Uses of Atomic Energy. Reactor Technology, Vol. 7 (1958), p. 223.
R. Bakish and F. Kern,Corrosion 16, 533t (1960).
H. W. Pickering, F. H. Beck, and M. A. Fontana,Trans. ASM 53, 793 (1961).
Patricia A. Alexander,The Mechanism of Corrosion by Fuel Impurities (Butterworth, London, 1963), p. 571.
A. Davin, V. LeRoy, D. Coutsouradis, and L. Habraken,Rev. Met. (Sci. Mem.) 66, 275 (1963).
Yu. Ugaste,Fiz., Metal. i Metalloved. 24 (3), 442 (1967).
M. Stainburg and N. H. Nachtrieb,J. Am. Chem. Soc. 72, 3358 (1960).
A. U. Barabashkin, M. V. Smirnow, and N. A. Saltykova,Physical Chemistry of Molten Salts and Slags [transl. of a Russian Conference, Moskow (1962)] AEC-tr-5948 (1960).
T. R. Anthony, G. E. Research & Development Center, Schenectady, N.Y., private communication.
W. T. Rees, B. D. Burns, and A. J. Cook,J. Iron Steel Inst. 162, 325 (1949).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Seybolt, A.U. Oxidation of Ni-20 Cr alloy and stainless steels in the presence of chlorides. Oxid Met 2, 119–143 (1970). https://doi.org/10.1007/BF00603652
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00603652