Summary
-
1.
We have used intracellular recording and staining, followed by reconstruction from serial sections, to characterize the responses and structure of projection neurons (PNs) that link the antennal lobe (AL) to other regions of the brain of the male sphinx mothManduca sexta.
-
2.
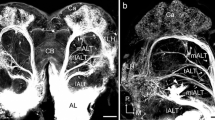
Dendritic arborizations of the AL PNs were usually restricted either to ordinary glomeruli or to the male-specific macroglomerular complex (MGC) within the AL neuropil. Dendritic fields in the MGC appeared to belong to distinct partitions within the MGC (Figs. 2, 3). PNs innervating the ordinary glomeruli had arborizations in a single glomerulus (uniglomerular) (Figs. 6, 7, 9, 11, 12A) or in more than one ordinary glomerulus of one AL (multiglomerular) (Figs. 12B, C, 14, 15), or in one case, in single glomeruli in both ALs (bilateral-uniglomerular) (Fig. 16). One PN innervated the MGC and many or all ordinary glomeruli of the AL (Fig. 13).
-
3.
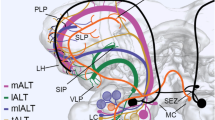
PNs with dendritic arborizations in the ordinary glomeruli and PNs associated with the MGC typically projected both to the calyces of the ipsilateral mushroom body and to the lateral protocerebrum, but some differences in the patterns of termination in those regions have been noted for the two classes of PNs (Figs. 2, 3, 6, 7, 9, 16). One PN conspicuously lacked branches in the calyces but did project to the lateral protocerebrum (Fig. 14). The PN innervating the MGC and many ordinary glomeruli projected to the calyces of the ipsilateral mushroom body and the superior protocerebrum (Fig. 13).
-
4.
Crude sex-pheromone extracts excited all neurons with arborizations in the MGC, although some were inhibited by other odors (Figs. 3, 4). One P(MGC) was excited by crude sex-pheromone extract and by a mimic of one component of the pheromone blend but was inhibited by another component of the blend (Fig. 5).
-
5.
PNs with dendritic arborizations in ordinary glomeruli were excited (Figs. 7, 8, 10) or inhibited (Figs. 9, 11) by certain non-pheromonal odors. Some of these PNs also responded to mechanosensory stimulation of the antennae (Figs. 10, 11, 15, 16).
-
6.
The PN with dendritic arborizations in the MGC and many ordinary glomeruli was excited by crude sex-pheromone extracts and non-pheromonal odors and also responded to mechanosensory stimulation of the antenna (Fig. 13).
Similar content being viewed by others
Abbreviations
- AL :
-
antennal lobe
- Ca :
-
primary calyces of the mushroom body
- cb :
-
cell body
- DACT :
-
dorsal antenno-cerebral tract
- DMACT :
-
dorsomedial antenno-cerebral tract
- FE :
-
female equivalent
- G :
-
ordinary glomerulus
- GABA :
-
γ-aminobutyric acid
- IACT :
-
inner antenno-cerebral tract
- ILPR :
-
inferior lateral protocerebrum
- L :
-
lateral
- LH :
-
lateral horn of the protocerebrum
- LN :
-
local interneuron
- MGC :
-
macroglomerular complex
- Oe :
-
oesophageal canal
- P :
-
posterior
- PD(G) :
-
PN with axon in DACT and arborizations in one or more AL ordinary glomeruli
- PDM(G) :
-
PN with axon in DMACT and arborizations in one or more AL ordinary glomeruli
- P(G) :
-
PN with arborizations in one or more AL ordinary glomeruli
- PIa(G) :
-
PN with axon in IACT, type a, and arborizations in one or more AL ordinary glomeruli
- PIa(MGC) :
-
PN with axon in IACT, type a, and arborizations in MGC
- PI(MGC, G) :
-
PN with axon in IACT and arborizations in MGC and one or more AL ordinary glomeruli
- P (MGC) :
-
PN with arborizations in MGC
- PN :
-
projection neuron
- SOG :
-
suboesophageal ganglion
- SPC :
-
superior protocerebrum
- LY :
-
Lucifer Yellow CH
- TES :
-
N-tris[hydroxymethyl]methyl-2-aminoethanesulfonic acid
References
Bell RA, Joachim FA (1976) Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms. Ann Entomol Soc Am 69:365–373
Boeckh J, Boeckh V (1979) Threshold and odor specificity of pheromone-sensitive neurons in the deutocerebrum ofAntheraea pernyi andA. polyphemus. J Comp Physiol 132:235–242
Boeckh J, Ernst KD (1987) Contribution of single unit analysisin insects to an understanding of olfactory function. J Comp Physiol A 161:549–565
Boeckh J, Boeckh V, Kühn A (1977) Further data on the topography and physiology of central olfactory neurones in insects. In: LeMagnen J, MacLeod P (eds) Olfaction and taste VI. Information retrieval, London Washington DC, pp 315–321
Boeckh J, Ernst KD, Sass H, Waldow U (1984) Anatomical and physiological characteristic of individual neurones in the central antennal pathway of insects. J Insect Physiol 30:15–26
Burrows M, Boeckh J, Esslen J (1982) Physiological and morphological properties of interneurones in the deutocerebrum of male cockroaches which respond to female pheromone. J Comp Physiol 145:447–457
Camazine SM, Hildebrand JG (1979) Central projections of antennal sensory neurons in mature and developingManduca sexta. Soc Neurosci Abstr 5:155
Christensen TA, Hildebrand JG (1987a) Male-specific, sex pheromone-selective projection neurons in the antennal lobes of the mothManduca sexta. J Comp Physiol A 160:553–569
Christensen TA, Hildebrand JG (1987b) Functions, organization, and physiology of the olfactory pathways in the lepidopteran brain. In: Gupta AP (ed) Arthropod brain: its evolution, development, structure and functions. Wiley, New York, pp 457–484
Christensen TA, Hildebrand JG, Tumlinson JH, Doolittle RE (in press) The sex pheromone blend ofManduca sexta: responses of central olfactory interneurons to antennal stimulation in male moths. Arch Insect Biochem Physiol
Christensen TA, Mustaparta H, Hildebrand JG (1989) Discrimination of sex pheromone blends in the olfactory system of the moth. Chemical Senses 14:202–217
Ernst K-D, Boeckh J (1983) A neuroanatomical study on the organization of the central antennal pathway in insects. III. Neuroanatomical characterization of physiologically defined response types of deutocerebral neurons inPeriplaneta americana. Cell Tissue Res 229:1–22
Ernst K-D, Boeckh J, Boeckh V (1977) A neuroanatomical study on the organization of the central antennal pathways in insects. II. Deutocerebral connections inLocusta migratoria andPeriplaneta americana. Cell Tissue Res 176:285–308
Harrow ID, Hildebrand JG (1982) Synaptic interactions in the olfactory lobe of the mothManduca sexta. Soc Neurosci Abstr 8:528
Hildebrand JG, Hall LM, Osmond BC (1979) Distribution of binding sites for125I-labeledα-bungarotoxin in normal and deafferented antennal lobes ofManduca sexta. Proc Natl Acad Sci USA 76:499–503
Hildebrand JG, Matsumoto SG, Camazine SM, Tolbert LP, Blank S, Ferguson H, Ecker V (1980) Organisation and physiology of antennal centres in the brain of the mothManduca sexta. In: Insect neurobiology and pesticide action (Neurotox 79). Soc Chem Ind, London, pp 375–382
Homberg U (1984) Processing of antennal information in extrinsic mushroom body neurons of the bee brain. J Comp Physiol A 154:825–836
Homberg U, Kingan TG, Hildebrand JG (1987) Gastrin/CCK-like peptides in the brain of the tobacco hawkmothManduca sexta. Soc Neurosci Abstr 13:235
Homberg U, Montague RA, Hildebrand JG (1988) Anatomy of antenno-cerebral pathway in the brain of the sphinx mothManduca sexta. Cell Tissue Res 254:255–281
Homberg U, Christensen TA, Hildebrand JG (1989) Structure and function of the deutocerebrum in insects. Annu Rev Entomol 34:477–501
Hoskins SG, Homberg U, Kingan TG, Christensen TA, Hildebrand JG (1986) Immunocytochemistry of GABA in the antennal lobes of the sphinx mothManduca sexta. Cell Tissue Res 244:243–252
Kaissling K-E, Hildebrand JG, Tumlinson JH (1989) Pheromone receptor cells in the male mothManduca sexta. Arch Insect Biochem Physiol, in press
Kanzaki R, Shibuya T (1986a) Identification of the deutocerebral neurons responding to the sexual pheromone in the male silkworm moth brain. Zool Sci 3:409–418
Kanzaki R, Shibuya T (1986b) Descending protocerebral neurons related to the mating dance of the male silkworm moth. Brain Res 377:378–382
Kanzaki R, Shibuya T (1987) Neuronal processing by central olfactory neurons related to the initiation of mating behavior in the male silkworm moth. Soc Neurosci Abstr 13:139
Keil TA (1989) Fine structure of the pheromone-sensitive sensilla on the antenna of the hawkmoth,Manduca sexta. Tissue Cell 21:139–151
Kent KS (1985) Metamorphosis of the antennal center and the influence of sensory innervation on the formation of glomeruli in the hawk mothManduca sexta. PhD Thesis, Harvard University, Cambridge MA
Matsumoto SG, Hildebrand JG (1981) Olfactory mechanisms in the mothManduca sexta: Response characteristics and morphology of central neurons in the antennal lobes. Proc R Soc Lond B 213:249–277
Mobbs PG (1982) The brain of the honeybeeApis mellifera. I. The connections and spatial organization of the mushroom bodies. Phil Trans R Soc Lond B 298:309–354
Olberg RM (1983) Interneurons sensitive to female pheromone in the deutocerebrum of the male silkworm moth,Bombyx mori. Physiol Entomol 8:419–428
Pearson L (1971) The corpora pedunculata ofSphinx ligustri L. and other Lepidoptera: an anatomical study. Proc R Soc Lond B 259:477–516
Roberts CJ, Krogsgaard-Larsen P, Walker RJ (1981) Studies of the action of GABA, muscimol and related compounds onPeriplaneta andLimulus central neurons. Comp Biochem Physiol 69C:7–11
Sanes JR, Hildebrand JG (1976a) Structure and development of antennae in a moth,Manduca sexta. Dev Biol 51:282–299
Sanes JR, Hildebrand JG (1976b) Origin and morphogenesis of sensory neurons in an insect antenna. Dev Biol 51:300–319
Schildberger K (1984) Multimodal interneurons in the cricket brain: Properties of identified extrinsic mushroom body cells. J Comp Physiol A 154:71–79
Schneiderman AM (1984) Postembryonic development of a sexually dimorphic sensory pathway in the central nervous system of the sphinx moth,Manduca sexta. PhD Thesis, Harvard University, Cambridge MA
Schneiderman AM, Hildebrand JG, Brennan MM, Tumlinson JH (1986) Transsexually grafted antennae alter pheromone-directed behavior in a moth. Nature 323:801–803
Schweitzer ES, Sanes JR, Hildebrand JG (1976) Ontogeny of electroantennogram responses of the moth,Manduca sexta. J Insect Physiol 22:955–960
Selzer LS (1984) Physiology and morphology of the larval sexual pheromone-sensitive neurones in the olfactory lobe of the cockroach,Periplaneta americana. J Insect Physiol 30:537–546
Selzer R (1979) Morphological and physiological identification of food odour specific neurones in the deutocerebrum ofPeriplaneta americana. J Comp Physiol 134:159–163
Spurr AR (1969) A low viscosity epoxyresin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Starrätt AM, Dahm KH, Allen N, Hildebrand JG, Payne TL, Roller H (1978) Bombykal, a sex pheromone of the sphinx moth,Manduca sexta. Z Naturforsch 34C:9–12
Strausfeld NJ, Seyan HS, Wohlers D, Bacon JP (1983) Lucifer yellow histology. In: Strausfeld NJ (ed) Functional neuroanatomy. Springer, Berlin Heidelberg New York, pp 132–155
Tolbert LP, Matsumoto SG, Hildebrand JG (1983) Development of synapses in the antennal lobes of the mothManduca sexta during metamorphosis. J Neurosci 3:1158–1175
Tumlinson JH, Brennan MM, Doolittle RE, Mitchell ER, Brabham A, Mazomenos BE, Baumhover AH, Jackson DM (in press) Identification of a pheromone blend attractive toManduca sexta (L.) males in a wind tunnel. Arch Insect Biochem Physiol
Usherwood PNR (1978) Amino acids as neurotransmitters. Adv Comp Physiol Biochem 7:227–309
Waldow U (1977) CNS units in cockroach (Periplaneta americana): Specificity of response to pheromones and other odor stimuli. J Comp Physiol 116:1–17
Waldrop B, Christensen TA, Hildebrand JG (1987) GABA-mediated synaptic inhibition of projection neurons in the antennal lobes of the sphinx moth,Manduca sexta. J Comp Physiol A 161:23–32
Yamada M (1971) A search for odour encoding in the olfactory lobe. J Physiol (Lond) 214:127–143
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kanzaki, R., Arbas, E.A., Strausfeld, N.J. et al. Physiology and morphology of projection neurons in the antennal lobe of the male mothManduca sexta . J. Comp. Physiol. 165, 427–453 (1989). https://doi.org/10.1007/BF00611233
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00611233