Abstract
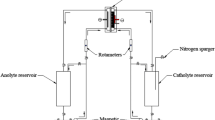
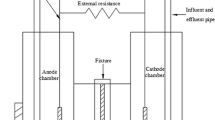
Mass transfer rates were measured at a single screen and a fixed bed of closely packed screens for the simultaneous cathodic reduction of K3Fe(CN)6 and anodic oxidation of K4Fe(CN)6 in alkaline solution with H2 and O2 evolution, respectively. Variables studied were gas discharge rate, number of screens per bed and position of the electrode (vertical and horizontal). For single screen electrodes, the mass transfer coefficient was related to the gas discharge rate by the equations:
. Electrode position was found to have no effect on the rate of mass transfer for single and multiscreen electrodes in the case of H2 and O2 evolution. Mass transfer coefficients were found to increase with an increasing number of screens per bed in the case of H2 evolution, while in the case of O2 evolution the mass transfer coefficient decreased with an increasing number of screens per bed. A mathematical model was formulated to account for the behaviour of the H2 evolving electrode which, unlike the O2 evolving electrode, did not obey the penetration model. Power consumption calculations have shown that the beneficial effect of mass transfer enhancement is outweighed by the increase in the voltage drop due to gas evolution in the bed electrode.
Similar content being viewed by others
Abbreviations
- a, ā :
-
constants
- A :
-
electrode area, cm2
- C :
-
concentration, mol cm−3
- D :
-
diffusivity, cm2s−1
- F :
-
Faraday's constant
- g :
-
gravitational acceleration cm s−2
- Gr :
-
Grashof number,Gr=(gL 3/v 2)(Δρ/\(\bar \rho\))
- i :
-
current, A
- K :
-
mass transfer coefficient, cm s−1
- L :
-
electrode length, cm
- Sc :
-
Schmidt number,Sc=μ/ρD
- Sh :
-
Sherwood number,Sh=KL/D
- V :
-
gas volume discharge rate, cm3 cm−2 min−1
- Z :
-
number of electrons in reaction
- ε :
-
void fraction
- μ :
-
viscosity, g cm−1 s−1
- v :
-
kinematic viscosity cm2 s−1
- ρ :
-
density, g cm−3
- g:
-
gas phase (subscript)
- l:
-
liquid phase (subscript)
References
A. A. Wragg and L. L. R. Whiteley, Extended Abstracts, 32nd Meeting of SIE, Dubrovnik, Yugoslavia, September 1981, p. 1106, V.II.
C. L. Lopez-Cacicedo,Trans. Inst. Met. Finishing 53 (1975) 74.
Idem, Inst. Chem. Eng. Symp. Ser. No. 42 (1975) 29.
Idem, Proceedings of the Symposium on Less Common Uses of Electricity in the Process Industries, Institution of Chemical Engineers, July (1979).
C. Chou and J. C. Chang,Chem. Eng. Sci. 35 (1980) 1581.
B. Surfleet and V. A. Crowle,Trans. Inst. Met. Finishing 50 (1972) 227.
L. W. Shemilt and G. H. Sedahmed,J. Appl. Electrochem. 6 (1976) 471.
G. H. Sedahmed and L. W. Shemilt, to be published.
A. A. Wragg,Int. J. Heat Mass Transfer 11 (1968) 979.
G. H. Sedahmed,J. Appl. Electrochem. 8 (1978) 399.
R. Alkire and B. Gracon,J. Electrochem. Soc. 122 (1975) 1594.
R. E. Sioda,Electrochim. Acta 15 (1970) 783.
Idem, ibid. 17 (1972) 1939.
Idem, ibid. 22 (1977) 439.
R. E. Sioda,J. Appl. Electrochem. 7 (1977) 135.
Idem, J. Electroanal. Chem. 34 (1972) 411.
A. Storck, P. M. Robertson and N. Ibl,Electrochim. Acta 24 (1979) 373.
J. Cano and U. Böhm,Chem. Eng. Sci. 32 (1977) 213.
M. A. Shah and D. Roberts, Advances in Chemistry, Series 133 — Chemical Reaction Engineering II (1974).
B. Gay and R. Maugham,Int. J. Heat Mass Transfer 6 (1963) 277.
C. N. Satterfield and D. H. Cortez,Ind. Eng. Chem Fundamentals 9 (1970) 613.
P. H. Vogtlander and C. A. P. Bakker,Chem. Eng. Sci. 18 (1963) 583.
E. Sutzkover, C. Zur and M. Ariel,Israel J. Chem. 18 (1979) 99.
W. J. Blaedel and S. L. Boyer,Anal. Chem. 45 (1973) 258.
M. G. Fouad and G. H. Sedahmed,Electrochim. Acta 20 (1975) 615.
N. Ibl, R. Kind and E. Adam,An. Quim. 71 (1975) 1008.
L. J. J. Janssen and J. G. Hoogland,Electrochim. Acta 18 (1973) 543.
Idem, ibid. 15 (1970) 1012.
N. Ibl and J. Venczel,Metalloberfläche 24 (1970) 365.
H. Vogt, PhD thesis, Stuttgart (1977).
A. I. Vogel, ‘A Textbook of Quantitative Analysis’, 2nd edn., Longmans (1960).
J. C. Armour and J. N. Cannon,AIChE J. 14 (1968) 415.
R. Alkire and P. Y. Lu,J. Electrochem. Soc. 126 (1979) 2118.
L. J. J. Janssen,Electrochim. Acta 23 (1978) 81.
L. J. J. Janssen and E. Barendrecht,ibid. 24 (1979) 693.
L. J. J. Janssen and S. J. D. Van Stralen,ibid. 26 (1981) 1011.
M. G. Fouad and G. H. Sedahmed,ibid. 17 (1972) 665.
Idem, ibid. 18 (1973) 55.
Idem, ibid. 19 (1974) 861.
G. H. Sedahmed, I. A. S. Mansour, A. A. Zatout and N. A. Abdel-Hay,J. Appl. Electrochem. 10 (1980) 543.
G. H. Sedahmed, A. A. Zatout, I. A. S. Mansour and N. A. Abdel-Hay,Surf. Technol. 11 (1980) 61.
J. Rousar and V. Cezner,Electrochim. Acta 20 (1975) 289.
P. V. Polyakov, V. M. Shestakov, V. V. Burnakin and Y. M. Ryabukhin,Electrokhimya 16 (1980) 685.
F. O. Mixon, W. Y. Chou and K. O. Beatty,Heat Transfer 56 (1960) 75.
J. R. Selman and C. W. Tobias,Advan. Chem. Eng. 10 (1978) 211.
N. Zuber,Int. J. Heat Mass Transfer 6 (1963) 53.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sedahmed, G.H., Shemilt, L.W. Mass transfer characteristics of electrochemical reactors employing gas evolving mesh electrodes. J Appl Electrochem 14, 123–130 (1984). https://doi.org/10.1007/BF00611269
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00611269