Summary
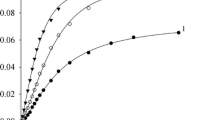
Rate constants are reported for the reaction of [PtCl4]2− with hydrochloric-perchloric acid mixtures, in aqueous methanol and aqueous t-butanol at 308.2 K. The observed first-order rate constants are, from their dependence on chloride concentration, divisible into forward and reverse rate constants for the equilibrium: [PtCl14]2−+H2O⇌[PtCl3(OH2)]−+Cl−. The solvent dependence of aquation rates for [PtCl4]2− is compared with those for other chlorotransition metal complexes, and discussed in terms of the Grunwald-Winstein method of mechanism diagnosis in organic systems. The solvent dependence of rates of [PtCl4]2− formation is compared with the rates of formation of other metal complexes; differences between this platinum reaction and, for example, nickel(II) formation, are rationalised in terms of the reactant charge product difference and consequent solvent permittivity effects on rate trends.
Similar content being viewed by others
References
A. A. Grinberg and Yu. N. Kukushkin,Zh. Neorg. Khim., 2, 2360 (1957); D. Banerjea, F. Basolo and R. G. Pearson,J. Am. Chem. Soc., 79, 4055 (1957); L. I. Elding and I. Leden,Acta Chem. Scand, 20, 706 (1966).
L. Drougge, L. I. Elding and L. Gustafson,Acta Chem. Scand., 21, 1647 (1967).
A. K. Johnson and J. D. Miller,Inorg. Chim. Acta, 16, 93 (1976).
Yu. N. Kukushkin and V. V. Kirillov,Russ. J. Inorg. Chem., 17, 1351 (1972).
K. A. Hoffman and G. Bugge,Chem. Ber., 41, 312 (1908).
J. Burgess,J. Chem. Soc. A. 2352 (1970).
L. F. Grantham, T. S. Ellemann and D. S. Martin,J. Am. Chem. Soc., 77, 2965 (1955).
E. Grunwald and S. Winstein,J. Am. Chem. Soc., 70, 846 (1948); P. R. Wells,Linear Free Energy Relationships, Academic Press, London, 1968, ch. 4.
V. D. Panasyuk and N. F. Malashok,Russ. J. Inorg. Chem., 14, 661 (1969).
V. D. Panasyuk and N. F. Malashok,Russ. J. Inorg. Chem., 14, 525 (1969).
L. A. P. Kane-Maguire and G. Thomas,J. Chem. Soc. Dalton Trans., 1890 (1975).
M. J. Blandamer, J. Burgess, M. Dupree and S. J. Hamshere,J. Chem. Research, (S)58, (M)0728 (1978).
J. Burgess,J. Chem. Soc. A, 2703 (1970); J. Burgess and M. G. Price,ibid., 3108 (1971).
J. Burgess, E. R. Gardner and F. M. Mekhail,J. Chem. Soc. Dalton Trans., 487 (1972).
J. Burgess,J. Chem. Soc. Dalton Trans., 825 (1973).
H. E. Brower, L. Hathaway and K. R. Brower,Inorg. Chem. 5, 1899 (1966).
H. Diebler and M. Eigen,Z. Phys. Chem. Frankf. Ausg., 20, 229 (1959); M. Eigen and K. Tamm,Z. Elektrochem., 66, 93, 107 (1962); R. G. Wilkins and M. Eigen,Adv. Chem. Ser., 49, 55 (1965); R. G. Wilkins,The Study of Kinetics and Mechanism of Reactions of Transition Metal Complexes, Allyn and Bacon, Boston, 1974, ch.4.
L. I. Elding,Inorg. Chim. Acta, 20, 65 (1976).
E. g., F. Basolo and R. G. Pearson,Mechanisms of Inorganic Reactions, 2nd Edit., Wiley, New York, 1967, ch. 6.
E.g., C. F. Wells,J. Chem. Soc. Faraday 1, 69, 984 (1973); 70, 694 (1974);71, 1868 (1975);72, 601 (1976).
G. Wada and Y. Kobayashi,Bull. Chem. Soc. Japan, 48, 2451 (1975).
R. J. Baltisberger, C. L. Knudson and M. F. Anderson,Inorg. Chem., 13, 2354 (1974).
W. J. MacKellar and D. B. Rorabacher,J. Am. Chem. Soc., 93, 4379 (1971); F. R. Shu and D. B. Rorabacher,Inorg. Chem., 11, 1496 (1972).
H. P. Bennetto and E. F. Caldin,J. Chem. Soc. A, 2207 (1971).
E. F. Caldin and P. Godfrey,J. Chem. Soc. Faraday I, 70, 2260 (1974).
H. L. Friedman and C. V. Krishnan, in F. Franks (Ed.),Water — A Comprehensive Treatise, Plenum Press, New York, 1973, vol. 3, ch. 1.
M. Born,Z. Phys., 1, 45 (1920).
M. J. Blandamer, J. Burgess and J. G. Chambers,J. Chem. Soc. Dalton, 60 (1977).
M. J. Blandamer and J. Burgess,Pure Appl. Chem., to be published.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Blandamer, M.J., Burgess, J. & Hamshere, S.J. Kinetics of solvolysis and of formation of tetrachloroplatinate(II) in alcohol: Water mixtures. Transition Met Chem 4, 291–294 (1979). https://doi.org/10.1007/BF00618317
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00618317