Summary
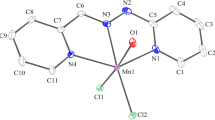
The chelation abilities of both pyridine-2-aldehyde semicarbazone (Pysc) and thiosemicarbazone (Pytsc) towards NiII and CoII salts have been investigated. A series of pentacoordinate monoligand chelates: Ni(Pysc)X2, Ni(Pytsc)X2 and Co(Pysc)X2 and octahedral bis ligand chelates Ni(Pysc)2X · NO3 Ni(Pytsc)2X2 and Co(Pysc)2X2 have been isolated and identified. In these chelates, both Pysc and Pytsc act as neutral tridentate ligands. The deprotonated form of Pyts, which arises through enolization, is identified in NII chelates and is of the type Ni(PytscH)2 and Ni(PytscH)X. Spectral and magnetic measurements are presented and attention is drawn to the effect of temperature on the solution spectra of some of the chelates.
Similar content being viewed by others
References
P. B. Danneberg, J. B. Montag and C. Heidelberger,Cancer Res., 18, 329 (1958).
F. A. French and E. J. Blanz,Cancer Res., 25, 1454 (1965);26, 1638 (1966a).
F. A. French and E. J. Blanz,J. Med. Chem., 9, 585 (1966).
F. A. French, E. J. Blanz, J. R. Doameral and D. A. French,J. Med. Chem., 13, 1117, 1124 (1970).
E. C. Moore and P. Reichard,J. Biol. Chem., 239, 3453 (1964).
F. A. French, A. E. Lewis, E. J. Blanz Jr. and A. H. Sheena,Fed. Proc. Fed. Am. Soc. Exp. Biol., 24, 402 (1965).
A. C. Sartorelli, Biochem.Biophys. Res. Comm., 27, 26 (1967).
F. A. French and B. L. Freedlander,Cancer Res., 18, 1290 (1958).
A. V. Ablov and N. I. Belick,Russ. J. Inorg. Chem., 14, 93 (1969); A. U. Ablov, N. V. Gerbeleu and B. T. Olio,ibid., 15, 1405 (1970), 99 (1971).
C. J. Jones and J. A. McCleverty,J. Chem. Soc. A, 38 (1971).
M. Mathew and G. J. Palenik,J. Am. Chem. Soc., 91, 6310 (1969).
M. A. Malik and D. J. Philips,J. Inorg. Nucl. Chem., 36, 2229 (1974),idem., Aust. J. Chem., 27, 1133 (1974).
M. Mashima,Bull. Chem. Soc. Japan, 37, 974 (1964); M. J. M. J. Campbell and R. Grzeskowiak,J. Chem. Soc. A, 396 (1967).
G. R. Burns,Inorg. Chem. 7, 277 (1968).
M. Schafer and C. Curran,Inorg. Chem., 5, 265 (1966); K. Swaminathan and H. M. N. H. Irving,J. Inorg. Nucl. Chem., 26, 1291 (1964).
C. H. Kline and J. Turkevich,J. Chem. Phys., 12, 300 (1944); N. S. Gill, R. H. Nuttell, D. E. Scaife and D. W. A. Sharp;J. Inorg. Nucl. Chem., 18, 79 (1961).
M. M. da Mota, J. Rodgers and S. M. Nelson,J. Chem. Soc. A, 2036 (1969); W. D. Dahlhoff and S. M. Nelson,J. Chem. Soc. A, 2184 (1971).
L. Sacconi,Pure Appl. Chem., 17, 95–127 (1968); M. Ciampolini,Structure and Bonding, 6, 52 (1969); C. Furlani,Coord. Chem. Rev., 3, 141 (1968).
M. Ciampolini and J. Gelsomini,Inorg. Chem., 6, 182 (1967).
L. Lions, I. G. Dance and J. Lewis,J. Chem. Soc. A, 565 (1967).
L. Sacconi in L. Carlin (Ed.),Transition Metal Chemistry, 4, 199 (1969).
W. J. Geary,Coord. Chem. Rev., 7, 81 (1971).
V. Gutmann in E. A. V. Ebsworth, A. G. Maddock and A. G. Sharpe (Eds.)New Pathways in Inorganic Chemistry, Cambridge Univ. Press, 1968.
N. S. Gill and R. S. Nyholm,J. Chem. Soc., 3997 (1959), M. Goodgame, D. M. L. Goodgame and F. A. Cotton,J. Am. Chem. Soc.,83, 4161 (1961).
A. B. P. Lever,Inorganic Electronic Spectroscopy, Elsevier, 1968.
T. N. Tarkhova, K. N. Akatova, A. V. Ablov and N. I. Belichuk,Dokl. Akad. Nauk, SSSR., 209, 124 (1973).
T. N. Tarkhova, K. N. Akatova and N. V. Belov,Kristallografiya, 19, 70 (1974); K. N. Akatova, T. N. Tarkhova, S. L. Ginzburg, M. G. Neigauz and L. A. Navakovskaya,Kristallografiya, 19, 383 (1974).
L. El-Sayed and M. F. Iskander,J. Inorg. Nucl. Chem., 33, 435 (1971).
M. F. Iskander, L. El-Sayed and M. A. Lasheen,Inorg. Chim. Acta, 16, 147 (1975).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Iskander, M.F., El-Sayed, L. & Zaki, K.I. Coordination compounds of hydrazine derivatives with transition metals, Part 17. Nickel (II) and cobalt(II) chelates with pyridine-2-aldehyde semi- and thiosemicarbazone. Transition Met Chem 4, 225–230 (1979). https://doi.org/10.1007/BF00619173
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00619173