Abstract
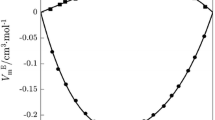
Enthalpies of dilution of propionamide, butyramide, pentanamide, hexanamide, N-pentylacetamide, N,N-dipentylacetamide, N-ethylhexanamide and N,N-diethylhexanamide dissolved in N,N-dimethylformamide as solvent have been measured calorimetrically at 25°C. The results are interpreted in terms of the McMillan-Mayer theory. Enthalpic interaction parameters are obtained for pairs, triplets and some quadruplets of solute molecules. All enthalpic pair interaction coefficients are negative, whereas those for triplets are positive. For unsubstituted amides the change of the enthalpic coefficients with the number of C-atoms differs considerably from that of the substituted compounds. The concept of polarophobic interaction is used for the interpretation of the results in connection with the assumption of formation of solute-solvent associates. For solutes with longer alkyl chains the results cannot be described satisfactorily in terms of the additivity approach of Savage and Wood. Probably the pair interactions of these compounds are not the result of interaction in a random way. Also the linear dependence of the pair interaction coefficients of the larger molecules with the number of C-atoms and the results for the unsubstituted amides support the occurrence of preferential orientations for these compounds.
Similar content being viewed by others
References
W. G. McMillan, Jr. and J. E. Mayer,J. Chem. Phys. 13, 276 (1945).
H. L. Friedman,Ionic Solution Theory (Interscience, New York, 1982).
T. L. Hill,An Introduction to Statistical Thermodynamics (Addison-Wesley, Reading, MA 1962) Chap. 19.
J. J. Kozak, W. S. Knight, and W. Kauzmann,J. Chem. Phys. 48, 675 (1968).
H. L. Friedman,J. Solution Chem. 1, 387, 413, 419 (1972).
R. B. Cassel and R. H. Wood,J. Phys. Chem. 78, 2460, 2465 (1974).
J. E. Desnoyers, G. Perron, L. Avéikian, and J.-P. Morel,J. Solution Chem. 5, 631 (1976).
F. Franks, M. Pedley, and D. S. Read,J. Chem. Soc. Faraday I 72, 359 (1976).
L. R. Pratt and D. Chandler,J. Solution Chem. 9, 1 (1980).
G. Barone, G. Castronuovo, and V. Elia,J. Solution Chem. 9, 607 (1980).
M. Bloemendal and G. Somsen,J. Solution Chem. 12, 83 (1983).
E. E. Schrier and E. B. Schrier,J. Phys. Chem. 71, 1851 (1967).
J. J. Savage and R. H. Wood,J. Solution Chem. 5, 733 (1976).
B. Y. Okamoto, R. H. Wood, and P. T. Thompson,J. Chem. Soc. Faraday I 74, 1990 (1978).
R. H. Wood and L. H. Hiltzik,J. Solution Chem. 9, 45 (1980).
A. C. Rouw and G. Somsen,J. Chem. Soc. Faraday I 78, 3397 (1982).
J. Vaughn and P. G. Sears,J. Phys. Chem. 62, 183 (1958).
P. K. Nandí and D. R. Robinson,J. Am. Chem. Soc. 94, 1299 (1972).
F. H. Verhoek,J. Am. Chem. Soc. 58, 2577 (1936).
J. C. Verhoef and E. Barendrecht,Anal. Chim. Acta 94, 395 (1977).
K. Heyns and W. von Bebenburg,Chem. Berichte 86, 278 (1953).
P. T. Thompson, D. E. Smith, and R. H. Wood,J. Chem. Eng. Data 19, 386 (1974).
G. M. Blackburn, T. H. Lilley, and E. Walmsley,J. Chem. Soc. Faraday I 76, 915 (1980).
W. J. M. Heuvelsland, C. de Visser, and G. Somsen,J. Chem. Soc. Faraday I 77, 1191 (1981).
A. C. Rouw, Ph.D. Thesis 1982, Free University, Amsterdam.
I. R. Tasker and R. H. Wood,J. Solution Chem. 11, 469, 729 (1982).
G. Barone, V. Elia, and E. Rizzo,J. Solution Chem. 11, 687 (1982).
I. R. Tasker and R. H. Wood,J. Solution Chem. 11, 481 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bloemendal, M., Somsen, G. Enthalpic interaction coefficients of amides dissolved in N,N-dimethylformamide. J Solution Chem 13, 281–295 (1984). https://doi.org/10.1007/BF00646401
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00646401