Abstract
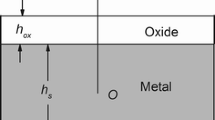
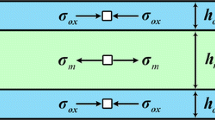
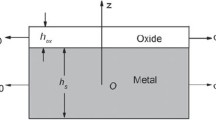
When an oxidized metal is cooled from a high temperature, stresses are produced at the metal-scale interface, owing to the difference in thermal expansion rates of the oxide and metal. Such stresses become time- and temperature-dependent if the scale or underlying metal creeps as cooling occurs. A model is presented which describes thermally induced stresses in the scale and accounts for partial stress relaxation by creep of the metal substrate and/ or the scale. The expected stresses are a function of the material parameters: thermal expansion coefficients, elastic modulii, and creep rates of both metal and scale. To illustrate a range of behaviors, we have presented example calculations for three Cr2O3 forming metals, Ni-30Cr, pure Cr, and MA-754. The effect of stress relaxation during thermal cycling was also examined briefly. In these examples, creep of the Cr2O3 scale was not expected to be important.
Similar content being viewed by others
Abbreviations
- A :
-
A constant evaluated from experimental creep data
- δ :
-
Grain-boundary thickness
- d :
-
Grain diameter
- Δα :
-
Thermal expansion difference;a m −a ox
- Deff, v, gb :
-
Diffusion coefficient; subscript eff, defined by Eq. (6)
- υ :
-
volume
- gb :
-
grain boundary
- ɛ:
-
Strain
- Eox,m :
-
Young's modulus; subscript
- ox:
-
oxide
- m :
-
metal
- G :
-
Shear modulus
- k :
-
Boltzmann constant
- n :
-
Stress-related exponent for power-law creep
- Q p, v, gb :
-
Activation energies for creep; subscript
- p :
-
power law
- v :
-
volume diffusion
- gb :
-
grain-boundary diffusion
- R :
-
Gas constant
- R :
-
Ratio of metal-to-oxide thickness
- σ s :
-
Shear stress
- T :
-
Temperature
- t :
-
Time
- t ox,m :
-
Thickness; subscript
- ox:
-
oxide
- m :
-
metal
- Ω:
-
Atomic volume of metal
- μ :
-
Poisson's ratio
References
J. Stringer,Corr. Sci. 10, 513 (1970).
P. Hancock and R. C. Hurst,Advances in Corrosion Science and Technology, M. G. Fontana and R. W. Staehle, eds. (Plenum Press, New York 1974), vol. 4, p. 1.
D. J. Baxter and K. N. Natesan,Rev. High Temp. Mat. 5, 149 (1983).
S. Taniguchi,Trans. Iron Steel Inst. Japan,25, 3 (1985).
J. H. Stout, W. W. Gerberich, S. Lin, and M. Lii, inFundamental Aspects of High Temperature Corrosion-II, D. A. Shores and G. J. Yurek, eds. (The Electrochemical Society, Pennington, New Jersey, 1986), p. 172.
A. M. Huntz and J. G. Zhao,Mat. Sci. Engr. 88, 213 (1987).
G. D. Oxx,Prod. Eng. 61–63 (1958).
J. K. Tien and J. M. Davidson, inStress Effects and the Oxidation of Metals, J. V. Cathcart, ed. (AIME, New York, 1975), p. 200.
D. L. Deadmore and C. E. Lowell,Oxid. Met. 11, 91 (1977).
J. D. Noden, C. J. Knights, and M. W. Thomas,Brit. Corros. J. 3, 47 (1968).
R. Rolls and J. H. Cleland,Phil Mag. A44, 943 (1981).
J. V. Cathcart and C. T. Liu,Oxid. Met. 6, 123 (1973).
J. H. Stout and S. J. Lin, private communication.
H. J. Frost and M. F. Ashby,Deformation-Mechanism Maps (Pergamon Press, New York 1982).
Y. S. Touloukian, R. K. Kirby, R. E. Taylor, and P. D. Desai,Thermophysical Properties of Matter: Thermal Expansion (Plenum Press, New York, 1975, 1977), vols. 12 and 13.
S. J. Rosenberg, ed.,Nickel and Its Alloys (N.B.S. Monograph 106, 1968).
Incomap Technical Package, Inco International, Huntington, West Virginia.
B. Burton and G. L. Reynolds,J. Mat. Sci. 13, 219 (1978).
J. R. Stephens and W. D. Klopp,J. Less-Comm. Met. 27, 87 (1972).
P. Shahinian and M. R. Achter,Trans. ASM 51, 244 (1959).
D. Sidey and B. Wilshire,Metal Science J. 3, 56 (1969).
T. E. Howson, J. E. Stulga, and J. K. Tien,Metall. Trans. A,11A, 1599 (1980).
J. D. Whittenberger,Metall. Trans. A 8A, 1155 (1977).
J. J. Barnes and D. A. Shores, to be published.
D. R. Holmes and R. T. Pascoe,Werkst. u. Korros. 10, 859 (1972).
C. C. Chang, R. S. Sandhu, R. L. Sierakowski, and W. E. Wolfe,Comp. Struct. 29, 783 (1988).
N. J. Pagano,Int. J. Solids Struct. 14, 385 (1978).
E. F. Rybicki,J. Composite Mat. 5, 354 (1971).
I. S. Raju and J. H. Crews,Comp. Struct. 14, 21 (1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barnes, J.J., Goedjen, J.G. & Shores, D.A. A Model for stress generation and relief in oxide — Metal systems during a temperature change. Oxid Met 32, 449–469 (1989). https://doi.org/10.1007/BF00665449
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00665449