Abstract
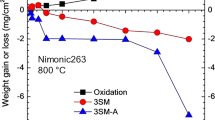
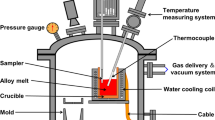
The corrosion behavior of Ni-Mo alloys containing up to 40 wt.% Mo was studied over the temperature range of 550–800‡C in a mixed gas of H2/H2O/ H2S. The scales formed on all alloys contained only sulfides and were doublelayered. The outer scale was single-phase Ni3S2. Depending on the alloy composition and reaction conditions, the inner scale was: (1) a mixture of MoS2 plus Ni3S2 with/without Ni, (2) MoS2, or (3) MoS2 plus intermetallic particles and/or double sulfide Ni2.5Mo6S6.7. Neither internal oxidation nor internal sulfidation were observed at lower temperatures. Internal sulfidation was however observed at higher temperature when the scale apparently melted. The parabolic law was generally obeyed for the most concentrated alloys. For the two more-dilute alloys the kinetics were mostly linear. A decrease in the corrosion rate occurred with increasing Mo content of the alloy and may be attributed to the presence of increasing volume fractions of MoS2 and/or of a double Ni-Mo sulfide in the inner region of the scale. For the two most concentrated alloys this may also be due to the presence of a number of particles of the unsulfidized intermetallic compound, which is Ni3Mo for Ni-30Mo, but NiMo for Ni-40Mo.
Similar content being viewed by others
References
S. Mrowec and K. Przybylski,High-Temp. Mater. Proc. 6, 1 (1984).
S. Mrowec and K. Przybylski,Oxid. Met. 23, 107 (1985).
S. Mrowec and J. Janowski, Nonstoichimetric compounds, Adv. Ceram.23, 585 (1987).
B. Gleeson, D. L. Douglass, and F. Gesmundo,Oxid. Met. 31, 209 (1989).
M. F. Chen and D. L. Douglass,Oxid. Met. 32, 185 (1989).
R. V. Carter, D. L. Douglass, and F. Gesmundo,Oxid. Met. 31, 341 (1989).
G. Wang, R. V. Carter, and D. L. Douglass,Oxid. Met. 31, 273 (1989).
M. F. Chen, D. L. Douglass, and F. Gesmundo,Oxid. Met. 31, 237 (1989).
B. Gleeson, D. L. Douglass, and F. Gesmundo,Oxid. Met. 33, 425 (1990).
F. Gesmundo, D. J. Young, and S. K. Roy,High-Temp. Mater. Proc. 8, 149 (1989).
P. Kofstad and G. Akesson,Oxid. Met. 12, 503 (1978).
M. Seiersten and P. Kofstad,Corros. Sci. 22, 497 (1982).
F. Gesmundo, C. de Asmundis, and P. Nanni,Oxid. Met. 20, 217 (1983).
C. S. Giggins and F. S. Pettit.Oxid. Met. 14, 363 (1980).
Reference data,J. Phys. Chem. 14, (Suppl.), 1516–1526 (1985).
Y. R. He, D. L. Douglass, and F. Gesmundo,Oxid. Met. (in press).
W. Kai, D. L. Douglass, and F. Gesmundo,Oxid. Met. 37, 389 (1992).
J. P. Orchard and D. Y. Young,J. Electrochem. Soc. 133, 1734 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
He, Y.R., Douglass, D.L. & Gesmundo, F. The corrosion behavior of Ni-Mo alloys in a H2/H2O/H2S gas mixture. Oxid Met 37, 413–439 (1992). https://doi.org/10.1007/BF00666628
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00666628