Abstract
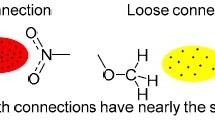
The crystal and molecular structure of nitrobenzene was determined at 103 K by X-ray diffraction, yieldingR=0.034 and a highly precise geometry of the molecule (esds of bond lengths ≤0.001 Å and bond angles ≤0.1°). The bond angles in the ring agree very well with additive scheme based on angular substituent parameters. X-X electron density maps support the view that the C-N bond does not exhibitπ-character, and, hence, the nitro group interacts with the ring mostly by inductive effects.
Similar content being viewed by others
References
Trotter, J.Acta Crystallogr. 1959,12, 884.
Høg, J. H.A study of nitrobenzene; Thesis, Univ. of Copenhagen, 1971; cited in Ref. [3].
Domenicano, A.; Schultz, G.; Hargittai, I.; Colapietro, M.; Portalone, G.; George, P.; Bock, C.Struct. Chem. 1990,1, 107.
Domenicano, A.; Murray-Rust, P.Tetrahedron Lett. 1979,24, 2283.
Norrestam, R.; Schepper, L.Acta Chem. Scand. 1981,35A, 91.
Shishkov, I. F.; Sadova, N. I.; Novikov, V. P.; Vilkov, L. V.Zh. Strukt. Khim. 1984,25(2), 98.
Catalano, D.; Forte, C.; Veracini, C. A.J. Magn. Resonan. 1984,60, 190.
Bock, C. W.; Trachtman, M.; George, P.J. Mol. Struct. (Theochem)1985,122, 155.
Krygowski, T. M.; Hafelinger, G.; Schule, J.Z. Naturforsch. 1986,41b, 895.
Huheey, J. E.J. Phys. Chem. 1965,69, 3284;1966,70, 2086.
Domenicano, A. InStereochemical Applications of Gas Phase Electron Diffraction, Ch. 7, Part B; I. Hargittai and M. Hargittai, Eds.; VCH: Weinheim, 1988; p. 282.
Further details of the crystal structure investigation are available on request from the Fachinformationszentrum Energie Physik Mathematik, D-7514 Eggenstein-Leopoldshafen 2; to request use the depository number CSD 320285, the authors names, and the full citation of the journal.
Brodalla, D.; Mootz, D.; Boese, R.; Osswald, W.J. Appl. Cryst. 1985,18, 316.
Cox, E. G.; Cruickshank, D. W. J.; Smith, J. A. S.Nature,1955,175, 766.
Kitaigorodsky, A.Molecular Crystals and Molecules; Academic Press, New York, 1973.
Dunitz, J. D.; Seiler, P.J. Am. Chem. Soc. 1983,105, 7056.
Coppens, P.Acta Crystallogr. 1984,A40, 184.
Augart, N. diploma work, University of Essen, 1986.
Krygowski, T. M.; Anulewicz, R.; Kruszewski, J.Acta Crystallogr. 1983,B29, 732.
Exner, O.Collect. Czech. Chem. Commun. 1966,31, 65.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boese, R., Bläser, D., Nussbaumer, M. et al. Low temperature crystal and molecular structure of nitrobenzene. Struct Chem 3, 363–368 (1992). https://doi.org/10.1007/BF00678559
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00678559