Summary
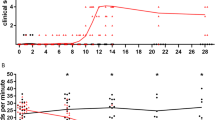
Two Icelandic sheep with clinical signs of visna appearing 6–7 years after intracerebral infection with visna virus were killed, fixed by perfusion and the central nervous system lesions examined by light and electron microscopy. Both sheep showed similar pathological changes. In the brain there was a severe periventricular inflammatory process with small foci of liquefaction necrosis and scattered small granulomas. In some areas of inflammation there was evidence of primary demyelination but it was not prominent. In the spinal cord there were focal plaques of primary demyelination. At the ultrastructural level the spinal cord lesions showed unambiguous primary demyelination with many naked axons; various stages of remyelination with peripheral type of myelin were also common.
These observations indicate that the CNS lesions of visna, as seen in Icelandic sheep, fall into two categories: (a) an inflammatory process which often begins within weeks of infection and which occurs in the majority of infected animals in the absence of clinical paresis; and (b) focal demyelinating lesions of the spinal cord which are seen in sheep with clinical paresis but are uncommon in animals prior to onset of clinical signs. Both types of lesions may coexist.
Similar content being viewed by others
References
Bradley R (1972) The post-mortem fixation of farm animals by vascular perfusion. Res Vet Sci 13:579–588
Brosnan CF, Stoner GL, Bloom BR, Wisniewski HM (1977) Studies on demyelination by activated lymphocytes in the rabbit eye. II. Antibody-dependent cell-mediated demyelination. J Immunol 118:2103–2110
Cork LC, Davis WC (1975) Ultrastructural features of viral leukoencephalomyelitis of goats. Lab Invest 32:359–365
Dal Canto MC, Lipton HL (1975) Primary demyelination in Theiler's virus infection. An ultrastructural study. Lab Invest 33:626–637
Dal Canto MC, Wisniewski HM, Johnson AB, Brostoff SW, Raine CS (1975) Vesicular disruption of myelin in autoimmune demyelination. J Neurol Sci 24:313–319
Dal Canto MC, Lipton HL (1977) Multiple sclerosis: Animal model: Theiler's virus infection in mice. Am J Pathol 88:497–500
Dal Canto MC, Lipton HL (1980) Schwann cell remyelination and recurrent demyelination in the central nervous system of mice infected with attenuated Theiler's virus. Am J Pathol 98:101–122
Dal Canto MC, Rabinowitz SG (1982) Experimental models of virus-induced demyelination of the central nervous system. Ann Neurol 11:128–135
Ghatak NR, Hirano A, Doron Y, Zimmerman HM (1973) Remyelination in multiple sclerosis with peripheral type myelin. Arch Neurol 29:262–267
Georgsson G, Pálsson PA, Panitch H, Nathanson N, Pétursson G (1977) The ultrastructure of early visna lesions. Acta Neuropathol (Berl) 37:127–135
Georgsson G, Martin JR, Pálsson PA, Nathanson N, Benediktsdóttir E, Pétursson G (1979) An ultrastructural study of the cerebrospinal fluid in visna. Acta Neuropathol (Berl) 48:39–43
Georgsson G, Nathanson N, Pétursson G, Martin JR, Pálsson PA (1980) Immunopathogenesis of visna. In: Clifford Rose F, Behan PO (eds) Animal models of neurological diseases. Pitman, Tunbridge Wells, pp 378–389
Haase AT, Stowring L, Narayan O, Griffin D, Price D (1977) Slow persistent infection caused by visna virus: role of host restriction. Science 195:175–177
Lampert PW (1967) Electron microscopic studies on ordinary and hyperacute experimental allergic encephalomyelitis. Acta Neuropathol (Berl) 9:99–126
Lampert PW, Sims JK, Kniazeff AJ (1973) Mechanism of demyelination in JHM virus encephalomyelitis. Electron microscopic studies. Acta Neuropathol (Berl) 24:76–85
Lipton HL (1975) Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun 11:1147–1155
Lumsden CE (1970) The neuropathology of multiple sclerosis. In: Vinken PJ, Bruyn GW (eds) Handbook of clinical neurology, vol 9. North-Holland, Amsterdam, pp 217–309
Martin JR, Nathanson N (1979) Animal models of virus-induced demyelination. In: Zimmerman HM (ed) Progress in neuropathology, vol 4. Raven Press, New York, pp 27–50
Nathanson N, Panitch H, Pálsson PA, Pétursson G, Georgsson G (1976) Pathogenesis of visna. II. Effect of immunosuppression upon early central nervous system lesions. Lab Invest 35:444–451
Nathanson N, Pétursson G, Georgsson G, Pálsson PA, Martin JR, Miller A (1979) Pathogenesis of visna. IV. Spinal fluid studies. J Neuropathol Exp Neurol 38:197–208
Nathanson N, Martin JR, Georgsson G, Pálsson PA, Lutley RE, Pétursson G (1981) The effect of post-infection immunization on the severity of experimental visna. J Comp Pathol 91:185–191
Ogata J, Feigin I (1975) Schwann cells and regenerated peripheral myelin in multiple sclerosis: An ultrastructural study. Neurology (Minneap) 25:713–716
Palay SL, Chan-Palay V (1974) Cerebellar cortex. Cytology and organization. Springer, Berlin Heidelberg New York
Panitch H, Pétursson G, Georgsson G, Pálsson PA, Nathanson N (1976) Pathogenesis of visna. III. Immune responses to central nervous system antigens in experimental allergic encephalomyelitis and visna. Lab Invest 35:452–460
Pétursson G, Nathanson N, Georgsson G, Panitch H, Pálsson PA (1976) Pathogenesis of visna. I. Sequential virologic, serologic and pathologic studies. Lab Invest 35:402–412
Powell HC, Lampert PW (1975) Oligodendrocytes and their myelinplasma membrane connections in JHM mouse hepatitis virus encephalomyelitis. Lab Invest 33:440–445
Prineas J (1975) Pathology of the early lesion in multiple sclerosis. Human Pathol 6:531–554
Raine CS, Snyder DH, Valsamis MP, Stone SH (1974) Chronic experimental allergic encephalomyelitis in inbred guinea pigs. An ultrastructural study. Lab Invest 31:369–380
Sigurdsson B, Pálsson PA (1958) Visna of sheep, a slow demyelinating infection. Br J Exp Pathol 39:519–528
Sigurdsson B, Pálsson PA, van Bogaert L (1962) Pathology of visna. Transmissible demyelinating disease in sheep in Iceland. Acta Neuropathol (Berl) 1:343–362
Snyder DH, Valsamis MP, Stone SH, Raine CS (1975) Progressive demyelination and reparative phenomena in chronic experimental allergic encephalomyelitis. J Neuropathol Exp Neurol 34:209–221
Whitaker JN (1977) Myelin encephalitogenic protein fragments in cerebrospinal fluid of persons with multiple sclerosis. Neurology (Minneap) 27:911–920
Wisniewski HM, Raine CS, Kay WJ (1972) Observations in viral demyelinating encephalomyelitis. Canine distemper. Lab Invest 26:589–599
Wisniewski HM, Bloom BR (1975) Primary demyelination as a nonspecific consequence of a cell-mediated immune reaction. J Exp Med 141:346–359
Wisniewski HM (1977) Immunopathology of demyelination in autoimmune diseases and virus infection. Br Med Bull 33:54–59
ZuRhein GM, Chou SM (1965) Particles resembling papovaviruses in human cerebral demyelinating diseases. Sciene 148:1477–1479
Author information
Authors and Affiliations
Additional information
Supported in part by NIH grant NS 16010
Rights and permissions
About this article
Cite this article
Georgsson, G., Martin, J.R., Klein, J. et al. Primary demyelination in visna. Acta Neuropathol 57, 171–178 (1982). https://doi.org/10.1007/BF00685386
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00685386