Abstract
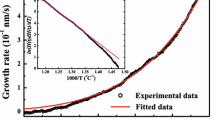
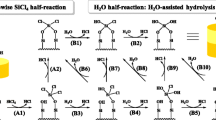
Zirconium dioxide (ZrO2) films have been deposited on to silicon wafers by the chemical vapour deposition (CVD) technique involving the application of gas mixtures of ZrCl4, CO2, and H2. The relationships between the deposition rate and various reaction parameters, such as the gas flow rate, the deposition temperature, and the composition of reactant gases, were studied. The film was identified as nearly stoichiometric monoclinic ZrO2 by using X-ray photoelectron spectroscopy, infrared transmission, and X-ray diffraction. Zirconium tetrachloride (ZrCl4) is the only species acting as zirconium donor which results from thermodynamic calculations in the present system. The CVD of ZrO2 is a thermally activated process and the activation energy is about 80 kJ mol−1 at the surface chemical reaction controlled region. The deposition mechanism, initially a kinetic process controlled by diffusive mass transfer, becomes a kinetic process governed by the surface chemical reactions with increasing total flow rate above 700°C. The dependence of the deposition rate on the reactant gas composition is mainly affected by the relative contents of the zirconium donor and the oxygen donor. At ZrCl4 mole fractions lower than 2.0 × 10−3, the deposition rate increases with the ZrCl4 mole fraction; however, at ZrCl4 mole fractions higher than that the deposition rate is mainly influenced by the H2O-forming reaction between CO2 and H2.
Similar content being viewed by others
References
E. S. SCHLEGEL,IEEE Trans. Elec. Devices ED-14 (1957) 728;ED-15 (1958) 951.
D. R. HARBISON and H. L. TAYLOR, in “Thin film dielectrics” edited by F. Vratny (Electrochemical Society, New York, 1959) pp. 254–271.
M. T. DUTTY, C. C. WANG, A. WAXMAN and K. H. ZAININGER,J. Electrochem. Soc. 116 (1969) 234.
G. WAHL, S. SCHLOSSER, F. SCHMADERER, in Proceedings, 7th International CVD Conference, Los Angeles, 1979, edited by T. O. Sedgewick and H. Lydtin (ECS Prineton, 1979) pp. 536–543.
K. S. MASDIYASNI and C. T. LYNCH, USAF Rep. ASD-TDR-63-322, May (1963).
C. F. POWELL, J. H. OXLEY and J. M. BLOCHER, Jr (eds), “Vapor deposition” (John Wiley, 1966) pp. 384–403.
N. IWAMOTO, Y. MAKINO and M. KAMAI,Thin Solid Films 153 (1987) 233–242.
M. SHIOJIRI, Y. HIROTA, T. ISSHIKI, T. MAEDA and S. SEKIMOTO, ibid.162 (1988) 235–246.
N. IWAMOTO and N. UMESAKI, ibid.127 (1985) 129–137.
K. BRENNFLECK, E. FIOTZER and G. MACK, in Proceedings, 8th International Conference CVD, edited by J. M. Blocher, G. E. Vvillard and E. Wahl (The Electrochemical Society, Pennington, 1981) p. 672.
K. F. SPEAR, in Proceedings, 7th International Conference CVD, Los Angeles, 1979, edited by T. O. Sedgewick and H. Lydtin (The Electrochemical Society, Princton, 1979).
W. B. WHITE, W. M. JOHNSON and G. B. DANTZIG,J. Chem. Phys. 28 (1958) 751–755.
JANAF Thermochemical Tables, NBS (1977).
N. T. McDEVITT and W. L. BAUN,J. Amer. Ceramic Soc. 47 (1964) 622.
D. W. SHAW, “Crystal growth”, p. 11 (Plenum Press, London, 1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Choi, JH., Kim, HG. & Yoon, SG. Effects of the reaction parameters on the deposition characteristics in ZrO2 CVD. J Mater Sci: Mater Electron 3, 87–92 (1992). https://doi.org/10.1007/BF00695722
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00695722