Abstract
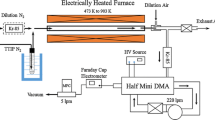
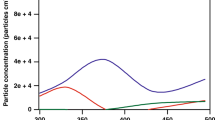
The growth of monodisperse particles (0.07 to 0.5 µm) exposed to SO2 (0–860 ppb), H2O2 (0–150 ppb) and sometimes NH3 (0–550 ppb) in purified air at 22 °C at relative humidities ranging from 25 to 75% were measured using the Tandem Differential Mobility Analyzer technique. The experiments were performed in a flow reactor with aqueous (NH4)2SO4 and Na2SO4 droplets. For (NH4)2SO4 droplets the fractional diameter growth was independent of size above 0.3 µm but decreased with decreasing size below that. When NH3 was added the fractional growth increased with decreasing size. Measurements were compared with predictions of a model that accounts for solubility of the reactive gases, the liquid phase oxidation of SO2 by H2O2, and ionic equilibria. Agreement between measured and predicted droplet growth is reasonable when the ionic strength effects are included. Theory and experiments suggest that NH3 evaporation is responsible for the decrease in relative growth rates for small aqueous ammonium sulfate particles. The observed droplet growth rates are too slow to explain observed growth rates of secondary atmospheric sulfate particles.
Similar content being viewed by others
References
Adkins, C. L. J., 1988, Use of continuous stirred tank reactor for the study of aqueous aerosol chemistry, Ph.D. Thesis, California Institute of Technology.
Bassett, M. and Seinfeld, J. H., 1983, Atmospheric equilibrium model of sulfate and nitrate aerosols,Atmos. Environ. 17, 2237–2252.
Bassett, M. and Seinfeld, J. H., 1984, Atmospheric equilibrium model of sulfate and nitrate aerosols — II. Particle size analysis,Atmos. Environ. 18, 1163–1170.
Berresheim, H. and Jaeschke, W., 1986, Study of metal aerosol systems as a sinks for atmospheric SO2,J. Atmos. Chem. 4, 311–334.
Bromley, L. A., 1973, Thermodynamic properties of strong electrolytes in aqueous solution,AlChE J. 19, 313–320.
Cain, P. W. and Carabine, M. D., 1978, Oxidation of sulfur dioxide in aerosol droplets, catalyzed by manganous sulfate,J. Chem. Soc. Faraday I 74, 2689–2702.
Calvert, J. G. and Stockwell, W. R., 1984, The mechanism and rates of the gas phase oxidations of sulfur dioxide and nitrogen oxides in the atmosphere, in J. G. Calvert (ed.),SO 2,NO, and NO 2 Oxidation Mechanism: Atmospheric Considerations, Butterworth, Boston, 1–62.
Clark, A. G. and Williams, P. T., 1983, The oxidation of sulphur dioxide in electrolyte droplets,Atmos. Environ. 17, 607–615.
Crump, J. G., Flagan, R. C., and Seinfeld, J. H., 1983, An experimental study of the oxidation of sulfur dioxide in aqueous manganese sulfate aerosols,Atmos. Environ. 17, 1277–1289.
Dlugi, R., 1983, SO2-oxidation in aerosol particles and droplets,J. Aerosol Sci. 14, 292–297.
Dlugi, R., Jordan, S., and Lindemann, E., 1981, The heterogeneous formation of sulfate aerosols in the atmosphere,J. Aerosol Sci. 12, 185–197.
Eigen, M., Kruse, W., Maass, G., and DeMager, L., 1964, Rate constants of photolytic reactions in aqueous solution, in G. Porter (ed.),Progress in Reaction Kinetics, Macmillan, New York, NY.
Freiberg, J. E. and Schwartz, S. E., 1981, Oxidation of SO2 in aqueous droplets: Mass-transport limitation in laboratory studies and the ambient atmosphere,Atmos. Environ. 15, 1145–1154.
Friedlander, S. K., 1977,Smoke, Dust and Haze: Fundamentals of Aerosol Behavior, John Wiley & Sons, New York.
Fuchs, N. A., 1963, On the stationary charge distribution of aerosol particles in a bipolar ionic atmosphere,Geofis. Pura. Appl. 56, 185–193.
Grgic', I., V. Hudnik, and M. Bizjak, J. Levec, 1993, Aqueous S(IV) oxidation-III. Catalytic effect of soot particles,Atmos. Environ. 27A, 1409–1416.
Gupta, A. and McMurry, P. H., 1989, A device for generating single charged particles in the 0.1–1.0 µm diameter range,Aerosol Sci. Technol. 10, 451–462.
Gupta, A., 1991, Experimental studies of gas-aerosol reactions,Ph.D. Thesis, Department of Mechanical Engineering, University of Minnesota, Minneapolis, MN 55455.
Haury, G., Jordan, S., and Hofmann, c., 1978, Experimental investigation of the aerosol-catalyzed oxidation of SO2 under atmospheric conditions,Atmos. Environ. 12, 281–287.
Heikes, B. G., 1984, Aqueous H2O2 production from O3 in glass impingers,Atmos. Environ. 18, 1433–1445.
Hering, S. V. and Friedlander, S. K., 1982, in Origins of aerosol sulfur size distributions in the Los Angeles basin,Atmospheric Environment 16, 2647–2656.
Hoffmann, M. R. and Edwards, J. O., 1975, Kinetics of the oxidation of sulfite by hydrogen peroxide in acidic solution,J. Phys. Chem. 79, 2096–2098.
John, W., Wall, S. M., Ondo, J. L., and Winklmayer, W., 1990, Modes in the size distributions of atmospheric inorganic aerosol,Atmos. Environ. 24A, 2349–2359.
Johnstone, H. F. and Leppla, P. W., 1934, The solubility of sulfur dioxide at low partial pressures. The ionization constant and heat of ionization of sulfurous acid.J. Am. Chem. Soc. 56, 2233–2238.
Kaplan, D. J., Himmelblau, D. M., and Kanaoka, C., 1981, Oxidation of sulfur dioxide in aqueous ammonium sulfate aerosols containing manganese as a catlayst,Atmos. Environ. 15, 763–773.
Knutson, E. and Whitby, K. T., 1975, Aerosol classification by electrical mobility: Apparatus, theory and applications,J. Aerosol Sci. 6, 443–451.
Kok, G. L., Holler, T. P., Lopez, M. B., and Natchtrieb, H. A., 1978, Chemiluminescent method for determination of hydrogen peroxide in the ambient atmosphere,Environ. Sci. Technol. 12, 1072–1076.
Kusik, C. L. and Meissner, H. P., 1978, Electrolyte activity coefficients in inorganic processing,A.I.Ch.E. Symp. 173, 14–20.
Lind, J. A. and Kok, G. L., 1986, Henry's law determinations of aqueous solutions of hydrogen peroxide, methylhydroperoxide, and peroxyacetic acid,J. Geophysical Research 91, 7889–7895.
Liu, B. Y. H. and Pui, D. Y. H., 1974, A submicron aerosol standard and the primary, absolute, calibration of the condensation nucleus counter,J. Colloid Interface Sci. 47, 155–171.
Martin, L. R. and Damschen, D. E., 1981, Aqueous oxidation of sulfur dioxide by hydrogen peroxide at low pH,Atmos. Environ. 15, 1615–1621.
Matteson, M. J., Stober, W., and Luther, H., 1969, Kinetics of the oxidation of sulfur dioxide by aerosols of manganese sulfate,Ind. Engr. Chem. Fund. 8, 677–687.
McArdle, J. V. and Hoffmann, M. R., 1983, Kinetics and mechanism of the oxidation of aquated sulfur dioxide by hydrogen peroxide at low pH,J. Phys. Chem. 87, 5425–5429.
McMurry, P. H. and Wilson, J. C., 1983, Droplet phase (heterogeneous) and gas phase (homogeneous) contributions to secondary ambient aerosol formation as functions of relative humidity,J. Geophysical Res. 88, 5101–5108.
McMurry, P. H., Takano, H., and Anderson, G. R., 1983, Study of the ammonia (gas) — sulfuric acid (aerosol) reaction rate,Environ. Sci. Technol. 17, 347–352.
Middleton, P., Kiang, C. S., and Mohnen, V. A., 1980, Theoretical estimates of the relative importance of various urban sulfate aerosol production mechanisms.Atmos. Environ. 14, 463–472.
Morgan, O. M. and Maass, O., 1931, An investigation of the equilibrium existing in gas-water systems forming electrolytes,Can. J. Res. 5, 162–199.
Penkett, S. A., Jones, B. M. R., Brice, K. A., and Eggleton, A. E. J., 1979, The importance of atmospheric ozone and hydrogen peroxide in oxidising sulphur dioxide in cloud and rainwater,Atmos. Environ. 13, 123–137.
Pitzer, K. S., 1979, Theory: Ion interaction approach, in R. M. Pytkowicz (ed.),Activity Coefficients in Electrolyte Solutions, Vol. 1, pp. 209–265, CRC Press, Boca Raton, Florida.
Rader, D. J., 1985, Application of the tandem differential mobility analyzer to studies of droplet evaporation and growth,Ph.D. Thesis, Mechanical Engineering Dept., University of Minnesota, Minneapolis, MN 55455.
Rader, D. J. and McMurry, P. H., 1986, Application of tandem differential mobility analyzer to studies of droplet growth or evaporation,J. Aerosol Sci. 17, 771–787.
Reilly, P. J., Wood, R. H., and Robinson, R. A., 1971, The prediction of osomotic and activity coefficients in mixed-electrolyte solutions,J. Phys. Chem. 75, 1305–1315.
Robinson, R. A. and Stokes, R. H., 1965,Electrolyte Solutions, Butterworth, London, U.K.
Rubow, K. K., 1981, Submicron aerosol filtration characteristics of membrane filter, Ph.D. Thesis, Mechanical Engineering Dept., University of Minnesota, Minneapolis, MN 55455.
Santachiara, G., Prodi, F., and Vivarelli, F., 1993, Further experiments on SO2 oxidation rate in monodisperse droplets grown on carbon nuclei in presence of O2 and NO2,J. Aerosol Sci. 24, 683–685.
Saxena, P. and Peterson, T. W., 1981, Thermodynamics of multicomponent electrolytic aerosols,J. Colloid & Interface Science 79, 496–510.
Saxena, P., Hydischewskyj, A. B., Seigneur, C., Seinfeld, J. H., 1986, A comparative study of equilibrium approaches to the chemical characterization of secondary aerosols,Atmos. Environ. 20, 1471–1483.
Saxena, P., Seigneur, C., and Peterson, T. W., 1983, Modeling of multiphase atmospheric aerosols,Atmos. Environ. 17, 1315–1329.
Scatchard, G., Rush, R. M., and Johnson, J. S., 1970, Osmostic and activity coefficients for binary mixtures of sodium chloride, sodium sulfate, magnesium sulfate, and magnesium chloride in water at 25 °C, III treatment with ions as components,J. Phys. Chem. 74, 3786–3796.
Schwartz, S. E. and Freiberg, J. E., 1981, Mass-transport limitation to the rate of reaction of gases in liquid droplets: Application to oxidation of SO2 in aqueous solutions,Atmos. Environ. 15, 1129–1144.
Schwartz, S. E., 1984, Gas aqueous reactions of sulfur and nitrogen oxides in liquid water clouds, in J. G. Calvert (ed.),SO 2,NO, and NO 2 Oxidation Mechanisms: Atmospheric Considerations, Butterworth, Boston, 173–208.
Seinfeld, J. H., 1986,Atmospheric Chemistry and Physics of Air Pollution, John Wiley & Sons, New York.
Stelson, A. W. and Seinfeld, J. H., 1982a, Relative humidity and temperature dependence of the ammonium nitrate dissociation constant,Atmos. Environ. 16, 983–992.
Stelson, A. W. and Seinfeld, J. H., 1982b, Relative humidity and pH dependence of the vapor pressure of ammonium nitrate-nitric acid solutions at 25 °C,Atmos. Environ. 16, 993–1000.
Stelson, A. W. and Seinfeld, J. H., 1982c, Thermodynamic prediction of the water activity, NH4NO3, dissociation constant, density and refractive index for the NH4NO3-(NH4)2SO4-H2O system at 25 °C,Atmos. Environ. 16, 2507–2514.
Stolzenburg, M. R., 1988, An ultrafine aerosol size distribution measuring system, Ph.D. Thesis, Mechanical Engineering Dept., University of Minnesota, Minneapolis, MN 55455.
Stolzenburg, M. R. and McMurry, P. H., 1991, An ultrafine aerosol condensation nucleus counter,Aerosol Sci. Technol. 14, 48–65.
Stumm, W. and Morgan, J. J., 1970,Aquatic Chemistry: An Introduction Emphasizing Chemical Equilibria in Natural Waters, Wiley-Interscience, New York.
Tang, I. N., 1976, Phase transformation and growth of aerosol particles composed of mixed salts,J. Aerosol Sci. 7, 361–371.
Tang, I. N. and Munkelwitz, H. R., 1984, An investigation of solute nucleation in levitated solution droplets,J. Colloid and Interface Sci. 98, 430–438.
Tang, I. N., Munkelwitz, H. R., and Davis, J. G., 1978, Aerosol growth studies-IV. Phase transformation of mixed salt aerosols in a moist atmosphere,J. Aerosol Sci. 9, 505–511.
Yui, T., 1940,Tokyo Inst. Phys. Chem. Res. Bull. 19, 1299.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gupta, A., Tang, D. & McMurry, P.H. Growth of monodisperse, submicron aerosol particles exposed to SO2, H2O2, and NH3 . J Atmos Chem 20, 117–139 (1995). https://doi.org/10.1007/BF00696554
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00696554