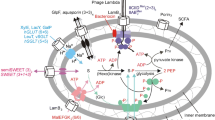
Abstract
The cell membranes of various bacteria contain proton-linked transport systems ford-xylose,l-arabinose,d-galactose,d-glucose,l-rhamnose,l-fucose, lactose, and melibiose. The melibiose transporter ofE. coli is linked to both Na+ and H+ translocation. The substrate and inhibitor specificities of the monosaccharide transporters are described. By locating, cloning, and sequencing the genes encoding the sugar/H+ transporters inE. coli, the primary sequences of the transport proteins have been deduced. Those for xylose/H+, arabinose/H+, and galactose/H+ transport are homologous to each other. Furthermore, they are just as similar to the primary sequences of the following: glucose transport proteins found in a Cyanobacterium, yeast, alga, rat, mouse, and man; proteins for transport of galactose, lactose, or maltose in species of yeast; and to a developmentally regulated protein of Leishmania for which a function is not yet established. Some of these proteins catalyze facilitated diffusion of the sugar without cation transport. From the alignments of the homologous amino acid sequences, predictions of common structural features can be made: there are likely to be twelve membrane-spanning α-helices, possibly in two groups of six, there is a central hydrophilic region, probably comprised largely of α-helix; the highly conserved amino acid residues (40–50 out of 472–522 total) form discrete patterns or motifs throughout the proteins that are presumably critical for substrate recognition and the molecular mechanism of transport. Some of these features are found also in other transport proteins for citrate, tetracycline, lactose, or melibiose, the primary sequences of which are not similar to each other or to the homologous series of transporters. The glucose/Na+ transporter of rabbit and man is different in primary sequence to all the other sugar transporters characterized, but it is homologous to the proline/Na+ transporter ofE. coli, and there is evidence for its structural similarity to glucose/H+ transporters in Plants.In vivo andin vitro mutagenesis of the lactose/H+ and melibiose/Na+ (H+) transporters ofE. coli has identified individual amino acid residues alterations of which affect sugar and/or cation recognition and parameters of transport. Most of the bacterial transport proteins have been identified and the lactose/H+ transporter has been purified. The directions of future investigations are discussed.
Similar content being viewed by others
References
Bachmann, B. J. (1987). InEscherichia coli and Salmonella typhimurium (Neidhardt, F. C., ed.), ASM, Washington, pp. 807–876.
Badia, J., Baldoma, L., Aguilar, J., and Boronat, A. (1989).FEMS Microbiol. Lett. 65, 253–258.
Baldwin, S. A., and Henderson, P. J. F. (1989).Annu. Rev. Physiol. 51, 459–471.
Baldwin, J. M., Lienhard, G. E., and Baldwin, S. A. (1980).Biochim. Biophys. Acta 599, 699–714.
Baldwin, S. A., Baldwin, J. M., and Lienhard, G. E. (1982).Biochemistry 21, 3836–3842.
Baly, D. L., and Horuk, R. (1988).Biochim. Biophys. Acta 947, 571–590.
Barnett, J. E. G., Holman, G. D., and Munday, K. A. (1973).Biochem. J. 131, 211–231.
Bassilana, M., Damiano-Ferano, E., and Leblanc, G. (1985).Biochem. Biophys. Res. Commun. 129, 626–631.
Beauclerk, A. D. D., and Smith, A. J. (1978).Eur. J. Biochem. 82, 187–197.
Beckwith, J. R., and Zipser, D. (1970).The Lactose Operon, Cold Spring Harbor Laboratory, New York.
Beyreuther, K., Bieseler, B., Ehring, R., and Muller-Hill, B. (1981). InMethods in Protein Sequence Analysis (Elzina, M., ed.), Humana Press, Clifton, New Jersey, pp. 139–148.
Birnbaum, M. J. (1989).Cell 57, 305–315.
Birnbaum, M. J., Haspel, H. C., and Rosen, O. M. (1986).Proc. Natl. Acad Sci. U.S.A. 83, 5784–5788.
Bloch, R. (1974).J. Biol. Chem. 249, 1814–1822.
Botfield, M. C., and Wilson, T. H. (1988).J. Biol. Chem. 263, 12909–12915.
Botfield, M. C., Wilson, D. M., and Wilson, T. H. (1990).Res. Microbiol. 141, 328–331.
Bradley, S. A., Tinsley, C. R., Muiry, J. A. R., and Henderson, P. J. F. (1987).Biochem. J. 248, 495–500.
Bremer, E., Silhavy, T. J., Weisemann, J. M., and Weinstock, G. M. (1984).J. Bacteriol. 158, 1084–1093.
Brooker, R. J. (1990).Res. Microbiol. 141, 309–315.
Brooker, R. J., and Wilson, T. H. (1985).Proc. Natl. Acad. Sci. USA 82, 3959–3963.
Broome-Smith, J. K., and Spratt, B. G. (1986).Gene 49, 341–349.
Buchel, D. E., Gronenborn, B., and Muller-Hill, B. (1980).Nature (London)283, 541–545.
Buttin (1968).Adv. Enzymol. Relat. Areas Mol. Biol. 30, 81–137.
Buvinger, W. E., and Riley, M. (1985).J. Bacteriol. 163, 850–857.
Cairns, M. T., Elliot, D. A., Scudder, P. R., and Baldwin, S. A. (1984).Biochem. J. 221, 179–188.
Cairns, M. T., Alvarez, J., Panico, M., Gibbs, A. F., Morris, H. R., Chapman, D., and Baldwin, S. A. (1987).Biochim. Biophys. Acta 905, 295–310.
Cairns, B. R., Collard, M. W., and Landfear, S. M. (1989a).Proc. Natl. Acad. Sci. USA 86, 7682–7686.
Cairns, M. T., Smith, G., Henderson, P. J. F., and Baldwin, S. A. (1989b).Biochem. Soc. Trans. 17, 552–553.
Carrasco, N., Herzlinger, D., Danho, W., and Kaback, H. R. (1986).Methods Enzymol. 125, 453–467.
Carter, J. R., Fox, C. F., and Kennedy, E. P. (1968).Proc. Natl. Acad. Sci. USA 60, 725–732.
Carter-Su, C., Pessin, J. E., Mora, R., Gitomer, W., and Czech, M. P. (1982).J. Biol. Chem. 257, 5419–5425.
Celenza, J. L., Marshall-Carlson, L., and Carlson, M. (1988).Proc. Natl. Acad. Sci. USA 85, 2130–2134.
Chang, Y.-D., and Dickson, R. C. (1988).J. Biol. Chem. 263, 16696–16703.
Charalambous, B. M., Maiden, M. C. J., McDonald, T. P., Cunningham, I. J., and Henderson, P. J. F. (1989).Biochem. Soc. Trans. 17, 441–444.
Ciaraldi, T. P., Horuk, R., and Matthaei, S. (1986).Biochem. J. 240, 115–123.
Cleland, W. W. (1963).Biochim. Biophys. Acta 67, 104–137.
Cleland, W. W. (1979).Methods Enzymol. 63, 103–138.
Cooper, R. A. (1986). InCarbohydrate Metabolism in Cultured Cells (Morgan, M. J., ed.), Plenum Press, London, pp. 461–491.
Cornish-Bowden, A. (1979).Fundamentals of Enzyme Kinetics, Butterworths, London.
Daie, J. (1989).Plant Mol. Biol. Rep. 7, 106–115.
Damiano-Forano, E., Bassilana, M., and Leblanc, G. (1986).J. Biol. Chem. 261, 6893–6899.
Daruwalla, K. R., Paxton, A. T., and Henderson, P. J. F. (1981).Biochem. J. 200, 611–627.
Davies, A., Meeran, K., Cairns, M. T., and Baldwin, S. A. (1987).J. Biol. Chem. 262, 9347–9352.
Davis, E. O. (1986). Ph.D. thesis, University of Cambridge.
Davis, E. O., and Henderson, P. J. F. (1987).J. Biol. Chem. 262, 13928–13932.
Davis, E. O., Jones-Mortimer, M. C., and Henderson, P. J. F. (1984).J. Biol. Chem. 259, 1520–1525.
Deves, R., and Krupka, R. M. (1978).Biochim. Biophys. Acta 510, 339–348.
Deziel, M., Pegg, W., Mack, E., Rothstein, A., and Klip, A. (1984).Biochim. Biophys. Acta 772, 403–406.
Diesenhofer, J., Epp, O., Miki, K., Huber, R., and Michel, H. (1985).Nature (London)318, 618–624.
Doolittle, R. F. (1981).Science 214, 149–159.
Dosch, D., Salvacion, F., and Epstein, W. (1984).J. Bacteriol. 160, 1188–1190.
Eckert, B., and Beck, C. F. (1989).J. Biol. Chem. 264, 11663–11670.
Eddy, A. A. (1982).Adv. Microbial. Physiol. 23, 1–78.
Eddy, A. A. (1989). In press.
Eiglmeier, K., Boos, W., and Cole, S. T. (1987).Mol. Microbiol. 1, 251–258.
Eisenberg, D. (1985).Annu. Rev. Biochem. 53, 595–623.
Eisenberg, D., Schwartz, E., Komaromy, M., and Wall, R. (1984).J. Mol. Biol. 179, 125–142.
Fersht, A. R. (1985).Enzyme Structure and Mechanism. Wiley, New York, pp. 317–331.
Flores, E., and Schmetterer, G. (1986).J. Bacteriol. 166, 693–696.
Foster, D. L., Boublik, M., and Kaback, H. R. (1983).J. Biol. Chem. 258, 31–34.
Fox, C. F., and Kennedy, E. P. (1965).Proc. Natl. Acad. Sci. USA 54, 891–899.
Friedrich, M. J., and Kadner, R. J. (1987).J. Bacteriol. 169, 3556–3563.
Fromm, H. J. (1979).Methods Enzymol. 63, 42–53.
Fukumoto, H., Kayano, T., Buse, J. B., Edwards, Y., Pilch, P. F., Bell, G. I., and Seino, S. (1989).J. Biol. Chem. 264, 7776–7779.
Fukumoto, H., Seino, S., Imura, H., Seino, Y., Eddy, R. L., Fukushima, Y., Byers, M. G., Shows, T. B., and Bell, G. I. (1988).Proc. Natl. Acad. Sci. USA 85, 5434–5438.
Furlong, C. E. (1987). InEscherichia coli and Salmonella typhimurium' (Neidhardt, F. C., ed.), ASM, Washington, pp. 768–796.
Garnier, J., Osguthorpe, D. J., and Rosen, B. (1978).J. Mol. Biol. 120, 97–120.
Gogarten, P. G., and Bentrup, F.-W. (1989).Planta 178, 52–60.
Griffin, J. F., Rampal, A. L., and Jung, C. Y. (1982).Proc. Natl. Acad. Sci. USA 79, 3759–3763.
Hanada, K., Yamato, I., and Anraku, Y. (1985).FEBS Lett. 191, 278–282.
Hanada, K., Yamato, I., and Anraku, Y. (1988a).Biochim. Biophys. Acta 939, 282–288.
Hanada, K., Yamato, I., and Anraku, Y. (1988b).J. Biol. Chem. 263, 7181–7185.
Haspel, H. C., Rosenfeld, M. G., and Rosen, O. M. (1988).J. Biol. Chem. 263, 398–403.
Hediger, M. A., Coady, M. J., Ikeda, T. S., and Wright, E. M. (1987).Nature (London)330, 379–381.
Hediger, M. A., Turk, E., and Wright, E. M. (1989).Proc. Natl. Acad. Sci. USA 86, 5748–5752.
Henderson, R., and Unwin, P. N. T. (1975).Nature (London)257, 28–32.
Henderson, P. J. F. (1985). InTechniques in Protein and Enzyme Biochemistry, Part II Supplement (Tipton, K. F., ed.), Elsevier, Dublin, pp. 1–48.
Henderson, P. J. F. (1986). InCarbohydrate Metabolism in Cultured Cells (Morgan, M. J., ed.), Plenum Press, London, pp. 409–460.
Henderson, P. J. F., and Kornberg, H. L. (1975). InEnergy Transformation in Biological systems, Ciba Foundation Symposium Vol. 31, Elsevier, Amsterdam, pp. 243–269.
Henderson, P. J. F., and Macpherson, A. J. S. (1986).Methods Enzymol. 125, 387–429.
Henderson, P. J. F., and Maiden, M. C. J. (1990).Philos. Trans. R. Soc. London Ser. B 326, 391–410.
Henderson, R., and Schertler, G. (1990).Philos. Trans. R. Soc. London Ser. B 326, 379–390.
Henderson, P. J. F., Giddens, R. A., and Jones-Mortimer, M. C. (1977).Biochem. J. 162, 309–320.
Henderson, P. J. F., Hirata, H., and Kagawa, Y. (1983a).Biochim. Biophys. Acta 732, 204–209.
Henderson, P. J. F., Hirata, H., Horne, P., Jones-Mortimer, M. C., Kaethner, T. E., and Macpherson, A. J. S. (1983b). InBiochemistry of Metabolic Processes (Lennon, D. L. F., Stratman, F. W., and Zahlten, R. N., eds.), Elsevier, New York, pp. 339–352.
Hickey, M. W., Hiller, A. J., and Jago, G. R. (1986).Appl. Environ. Microbiol. 51, 825–831.
Higgins, C. J. (1989).Nature (London)341, 103.
Higgins, C. F., Gallagher, M. P., Hyde, S. C., Mimmack, M. L., and Pearce, S. R. (1990).Philos. Trans. R. Soc. London Ser. B 326, 353–366.
Hillen, W., and Schollmeier, K. (1983).Nucleic Acids Res. 11, 525–539.
Holman, G. D., and Rees, W. D. (1987).Biochim. Biophys. Acta 897, 395–405.
Horne, P. (1980). Ph.D. Thesis, University of Cambridge.
Horne, P., and Henderson, P. J. F. (1983).Biochem. J. 210, 699–705.
Hutchings, V. (1978a).Planta 138, 229–235.
Hutchings, V. (1978b).Planta 138, 237–241.
Ishiguro, N., and Sato, G. (1985).J. Bacteriol. 164, 977–982.
James, D. E., Strube, M., and Mueckler, M. (1989).Nature (London)338, 83–87.
Jones, T. D. H., and Kennedy, E. P. (1969).J. Biol. Chem. 244, 5981–5987.
Jones-Mortimer, M. C., and Henderson, P. J. F. (1986).Methods Enzymol. 125, 157–180.
Joset, F., Buchou, T., Zhang, C.-C., and Jeanjean, R. (1988).Arch. Microbiol. 149, 417–421.
Jund, R., Weber, E., and Chevallier, M.-R. (1988).Eur. J. Biochem. 171, 417–424.
Jung, C. Y., and Rampal, A. L. (1977).J. Biol. Chem. 252, 5456–5463.
Kaback, H. R. (1972).Biochim. Biophys. Acta 265, 367–416.
Kaback, H. R. (1974).Science 186, 882–892.
Kaback, H. R. (1986).Ann. N.Y. Acad. Sci. 456, 291–304.
Kaback, H. R. (1987).Biochemistry 26, 2071–2076.
Kaback, H. R. (1989).Harvey Lect. 83, 77–105.
Kaback, H. R. (1990).Philos. Trans. R. Soc. London Ser. B 326, 425–436.
Kaczorowski, G. J., Leblanc, G., and Kaback, H. R. (1980).Proc. Natl. Acad. Sci. USA 77, 6319–6323.
Kaestner, K. M., Christy, R. J., McLenithan, J. C., Braiterman, L. T., Cornelius, P., Pekala, P. M., and Lane, M. D. (1989).Proc. Natl. Acad. Sci. USA 86, 3150–3154.
Karim, A. R., Rees, W. D., and Holman, G. D. (1987).Biochim. Biophys. Acta 902, 402–405.
Kartner, N. and Ling, V. (1989).Sci. Am., March, 26–33.
Kasahara, M., and Hinkle, P. (1977).J. Biol. Chem. 252, 7384–7390.
Kayano, T., Fukumoto, H., Eddy, R. L., Fan, Y. S., Byers, M. G., Shows, T. B., and Bell, G. I. (1988).J. Biol. Chem. 263, 15245–15248.
Kennedy, E. P. (1970). InThe Lactose Operon (Beckwith, J. R., and Zipser, D., eds.), Cold Spring Harbor, New York, pp. 49–92.
King, S. C., and Wilson, T. H. (1989a).J. Biol. Chem. 264, 7390–7394.
King, S. C., and Wilson, T. H. (1989b).Biochim. Biophys. Acta 982, 253–264.
Kohara, Y., Akiyama, K., and Isono, K. (1987).Cell 50, 495–508.
Kolodrubetz, D., and Schleif, R. F. (1981).J. Mol. Biol. 151, 215–227.
Komor, E., and Tanner, W. (1974).Eur. J. Biochem. 44, 219–223.
Kosiba, B. E., and Schleif, R. F. (1982).J. Mol. Biol. 156, 53–56.
Kornberg, H. L. (1990).Philos. Trans. R. Soc. London Ser. B 326, 505–514.
Kotyk, A. (1983).J. Bioenerg. Biomembr. 15, 307–319.
Kyte, J., and Doolittle, R. F. (1982).J. Mol. Biol. 157, 105–132.
Lam, V. M. S., Daruwalla, K. D., Henderson, P. J. F., and Jones-Mortimer, M. C. J. (1980).J. Bacteriol. 143, 396–402.
Leblanc, G., Pourcher, T., and Bassilana, M. (1989).Biochimie 71, 969–979.
Lefevre, P. G. (1961).Pharmacol. Rev. 13, 39–70.
Lengeler, J. W., Titgemeyer, F., Vogler, A. P., and Wohrl, B. M. (1990).Philos. Trans. R. Soc. London Ser. B 326, 489–504.
Levy, S. (1984). InAntimicrobial Drug Resistance (Brian, L. E., ed.), Academic Press, New York, pp. 191–240.
Levy, S. (1988).ASM News 54, 418–421.
Lin, E. C. C. (1987). InEscherichia coli and Salmonella typhimurium (Neidhardt, F. C., ed.), ASM, Washington, pp. 244–284.
Lodish, H. F. (1988).Trends Biochem. Sci. 13, 332–334.
Lombardi, F. J. (1981).Biochim. Biophys. Acta 649, 661–679.
Lowe, A. G., and Walmsley, A. R. (1986).Biochim. Biophys. Acta 857, 146–154.
Lu, Z., and Lin, E. C. C. (1989).Nucleic Acids Res. 17, 4883–4884.
Macpherson, A. J. S., Jones-Mortimer, M. C., and Henderson, P. J. F. (1981).Biochem. J. 196, 269–283.
Macpherson, A. J. S., Jones-Mortimer, M. C., Horne, P., and Henderson, P. J. F. (1983).J. Biol. Chem. 258, 4390–4396.
Maiden, M. C. J. (1987). Ph.D. thesis, University of Cambridge.
Maiden, M. C. J., Davis, E. O., Baldwin, S. A., Moore, D. C. M., and Henderson, P. J. F. (1987).Nature (London)325, 641–643.
Maiden, M. C. J., Jones-Mortimer, M. C., and Henderson, P. J. F. (1988).J. Biol. Chem. 263, 8003–8010.
Maloney, P. C. (1990).Philos. Trans. R. Soc. London Ser. B 326, 437–454.
Manoil, C., and Beckwith, J. (1986).Science 233, 1403–1408.
Markgraf, M., Bocklage, H., and Muller-Hill, B. (1985).Mol. Gen. Genet. 198, 473–475.
McKeown, B. J. (1988). CPGS thesis, University of Cambridge.
McMorrow, I., Chin, D. T., Fiebig, K., Pierce, J. L., Wilson, D. M., Reeve, E. C. R., and Wilson, T. H. (1988).Biochim. Biophys. Acta 945, 315–323.
McMurry, L. M., Petrucci, R. R., and Levy, S. B. (1980).Proc. Natl. Acad. Sci. USA 77, 3974–3977.
Menick, D., Lee, J. A., Brooker, R. J., Wilson, T. H., and Kaback, H. R. (1987).Biochemistry 26, 1132–1136.
Mitchell, P. (1961).Nature (London)191, 144–148.
Mitchell, P. (1963).Biochem. Soc. Symp. 22, 142–169.
Mitchell, P. (1973).J. Bioenerg. 4, 63–91.
Mueckler, M., Caruso, C., Baldwin, S. A., Panico, M., Blench, I., Morris, H. R., Allard, W. J., Leinhard, G. E., and Lodish, H. (1985).Science 229, 941–945.
Muiry, J. A. R. (1989). Ph.D. Thesis, University of Cambridge.
Nakao, T., Yamato, I., and Anraku, Y. (1987).Mol. Gen. Genet. 208, 70–75.
Newman, M. J., and Wilson, T. H. (1980).J. Biol. Chem. 255, 10583–10586.
Newman, M. J., Foster, D. L., Wilson, T. H., and Kaback, H. R. (1981).J. Biol. Chem. 256, 11804–11808.
Nordlie, R. C. (1985). InMetabolic Regulation (Ochs, R. S., Hanson, R. W., and Hall, J., eds.), Elsevier, Amsterdam, pp. 60–69.
Overath, P., Weigel, U., Neuhaus, J.-M., Soppa, J., Seckler, R., Riede, I., Bocklage, H., Muller-Hill, B., Aichelle, G., and Wright, J. K. (1987).Proc. Natl. Acad. Sci. USA 84, 5535–5539.
Page, M. G., and Rosenbusch, J. P. (1988).J. Biol. Chem. 263, 15906–15914.
Page, M. G., Rosenbusch, J. P., and Yamato, I. (1988).J. Biol. Chem. 263, 15897–15905.
Patel, L., Garcia, M. L., and Kaback, H. R. (1982).Biochemistry 21, 5805–5810.
Peden, K. W. (1983).Gene 22, 277–280.
Petro (1988). M. Philos. Thesis, University of Cambridge.
Poolman, B., Royer, T. J., Mainzer, S. E., and Schmidt, B. F. (1989).J. Bacteriol. 171, 244–253.
Postma, P. W., and Lengeler, J. W. (1985).Microbiol. Rev. 49, 232–269.
Pourcher, T., Bassilana, M., Sarkar, H. K., Kaback, H. R., and Leblanc, G. (1990).Philos. Trans. R. Soc. London Ser. B 326, 411–424.
Quiocho, F. A. (1986).Annu. Rev. Biochem. 55, 287–315.
Quiocho, F. A. (1990).Philos. Trans. R. Soc. London. Ser. B 326, 341–352.
Raboy, B., and Padan, E. (1978).J. Biol. Chem. 253, 3287–3291.
Rampal, A. L., and Jung, C. Y. (1987).Biochim. Biophys. Acta 896, 287–294.
Rausch, T., Raszeja-Specht, A., and Koepsell, H. (1989).Biochim. Biophys. Acta 985, 133–138.
Rees, W. D., and Holman, G. D. (1981).Biochim. Biophys. Acta 646, 251–260.
Reynolds, C. H., and Silver, S. (1983).J. Bacteriol. 156, 1019–1024.
Riordan, C., and Kornberg, H. L. (1977).Proc. R. Soc. London Ser. B 198, 401–410.
Riordanet al. (1989).Science 245, 1066–1073.
Rippka, R., Deruelles, J., Waterbury, J. B., Herdman, M., and Stanier, R. Y. (1979).J. Gen. Microbiol. 111, 1–61.
Roepe, P. D., and Kaback, H. R. (1989).Proc. Natl. Acad. Sci. USA 86, 6087–6091.
Roepe, P. D., Consler, T. J., Menezes, M. E., and Kaback, H. R. (1990).Res. Microbiol. 141, 290–308.
Romano, A. H. (1986). InCarbohydrate Metabolism in Cultured Cells (Morgan, M. J., ed.), Plenum Press, London, pp. 225–244.
Saier, M. H. (1985).Mechanisms and Regulation of Carbohydrate Transport in Bacteria, Academic Press, New York.
Sasatsu, M., Misra, T. K., Chu, L., Ladagu, R., and Silver, S. (1985).J. Bacteriol. 164, 983–993.
Sauer, N., and Tanner, W. (1989).FEBS Lett. 259, 43–46.
Severin, J., Langel, P., and Hofer, M. (1989).J. Bioenerg. Biomembr. 21, 321–334.
Shanahan, M. F. (1982).J. Biol. Chem. 257, 7290–7293.
Silhavy, T. J., Berman, M. L., and Enquist, L. W. (1984).Experiments with Gene Fusions, Cold Spring Harbor Laboratory, New York.
Stein, W. (1986).Transport and Diffusion across Cell Membranes, Academic Press, Orlando, Florida.
Stoker, N. G., Pratt, J. M., and Holland, I. B. (1984). InTranscription and Translation—A Practical Approach (Hames, B. D., and Higgins, S. J., eds.), IRL Press, Oxford, pp. 153–177.
Stoner, C., and Schleif, R. F. (1983).J. Mol. Biol. 171, 369–381.
Sumiya, M. (1989). Ph.D. thesis, University of Cambridge.
Szkutnicka, K., Tschopp, J. F., Andrews, L., and Cirillo, V. P. (1989).J. Bacteriol. 171, 4486–4493.
Tabor, S., and Richardson, C. C. (1985).Proc. Natl. Acad. Sci. USA 82, 1074–1079.
Teather, R. M., Muller-Hill, B., Abrutsch, U., Aichele, G., and Overath, P. (1978).Mol. Gen. Genet. 159, 239–248.
Thorens, B., Sarkar, H. K., Kaback, H. R., and Lodish, H. F. (1988).Cell 55, 281–291.
Tobin, J. F., and Schleif, R. F. (1987).J. Mol. Biol. 196, 789–799.
Tsuchiya, T., Ottina, K., Moriyama, Y., Newman, M. J., and Wilson, T. H. (1982).J. Biol. Chem. 257, 5125–5128.
van Dijken, J. P., and Scheffers, W. A. (1986).FEMS Microbiol. Rev. 32, 199–224.
Viitanen, P., Newman, M. J., Foster, D. L., Wilson, T. H., and Kaback, H. R. (1986).Methods Enzymol. 125, 429–452.
Vilaro, S., Palacin, M., Pilch, P. F., Testar, X., and Zorzano, A. (1989).Nature (London)342, 798–800.
von Heijne, G. (1987).Sequence Analysis in Molecular Biology—Treasure Trove or Trivial Pursuit. Academic Press, London.
Von Heijne, G. (1988).Biochim. Biophys. Acta 947, 307–333.
Walmsley, A. R. (1988).Trends Biochem. Sci. 13, 226–231.
Waters, S. H., Rogowsky, J., Grinstead, J., Altenbuchner, J., and Schmitt, R. (1983).Nucleic Acids Res. 11, 6089–6140.
West, I. C., (1970).Biochem. Biophys. Res. Commun. 41, 655–661.
West, I. C., and Mitchell, P. (1972).J. Bioenerg. 3, 445–462.
West, I. C., and Mitchell, P. (1973).Biochem. J. 132, 587–592.
Wheeler, T. J., and Hinkle, P. C. (1985).Annu. Rev. Physiol. 47, 503–517.
Wilson, D. M., and Wilson, T. H. (1987).Biochim. Biophys. Acta 904, 191–200.
Wilson, D. M., Tsuchiya, T., and Wilson, T. H. (1986).Methods Enzymol. 125, 377–387.
Wright, J. K., Teather, R. M., and Overath, P. (1983).Methods Enzymol. 97, 158–175.
Yao, B., Sollitti, P., and Marmur, J. (1989).Gene 79, 189–197.
Yazyu, H., Shiota-Niija, S., Shimamoto, T., Kanazawa, H., Futai, M., and Tsuchiya, T. (1984).J. Biol. Chem. 259, 4320–4326.
Yazyu, H., Shiota-Niija, S., Futai, M., and Tsuchiya, T. (1985).J. Bacteriol. 162, 933–937.
Zhang, C.-C., Durand, M.-C., Jeanjean, R., and Joset, F. (1989).Mol. Microbiol. 3, 1221–1229.
Zilberstein, D., and Dwyer, D. M. (1985).Proc. Natl. Acad. Sci. USA 82, 1716–1720.
Zilberstein, D., Dwyer, D. M., Matthei, S., and Horuk, R. (1986).J. Biol. Chem. 261, 15053–15057.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Henderson, P.J.F. Proton-linked sugar transport systems in bacteria. J Bioenerg Biomembr 22, 525–569 (1990). https://doi.org/10.1007/BF00762961
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00762961