Summary
-
1.
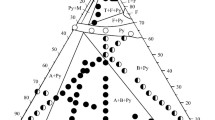
A new phase diagram is proposed for the system Y2O3-ZrO2 in the solid phase, differing from that of Duwez and co-authors by the presence of a compound Y2Zr2O7, by the absence of a single-phase region of the monoclinic solid solution, and also by the different position of the phase boundaries of the solid solutions in the system.
-
2.
The minimum amount of Y2O3 needed for complete stabilization of ZrO2 depends to a considerable extent on the roasting temperature.
-
3.
The reduction in the temperature of the polymorphic transformation of zirconium dioxide roasted with small additions of stabilizing oxides can be explained by the change in the repulsive force between the cations in the ZrO2 lattice due to the formation of a monoclinic substitutional solid solution of zirconium dioxide, the degree of reduction in the temperature of polymorphism depending on the size of this change.
Similar content being viewed by others
Literature cited
W. Nernst, Elektrochem.7, 373 (1901).
C. Tingwald, Phys. Z.36, 672 (1935).
Fan Fu-kang, A. K. Kuznetsov, and É.K. Keler, Izv. AN SSSR Otd. khim. n. 1962, 1141.
R. S. Roth, J. Res. Nat. Bur. Standards56, No. 1, 17 (1956).
P. Duwez, F. N. Brown, and F. Odell, J. Electtochem. Soc.98, No. 9, 356 (1951).
H. Hund, Z. Elektrochem. B55, 353 (1951).
C. Schusterius and N. N. Padurow, Ber. Dtsch. Keram. Ges.30, 235 (1953).
R. Collongues, M. Ferez, V. Jorda, and J. Lefevre, Bull. Soc. chim. France1, 70 (1961).
R. F. Geller and P. J. Javorsky, J. Res. Nat. Bur. Standards,35, 87 (1945).
G. B. Bokii, Crystal Chemistry [in Russian], Izd. Mosk. univ. (1960).
Author information
Authors and Affiliations
Additional information
This article is published on the instructions of a conference of the main editors of journals of the Academy of Sciences of the USSR, dated June 12, 1962, as the dissertation work of Fan Fu-kang.
Rights and permissions
About this article
Cite this article
Fu-kang, F., Kuznetsov, A.K. & Keler, É.K. Phase relationships in the system Y2O3-ZrO2 . Russ Chem Bull 12, 542–549 (1963). https://doi.org/10.1007/BF00843937
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00843937