Abstract
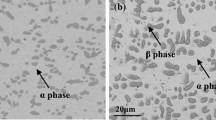
The sulfidation behavior of Co-Nb alloys containing up to 30wt.% Nb was studied in sulfur vapor at a pressure of 0.01 atm in the temperature range of 600–700°C. Increasing niobium content decreased the sulfidation rate, following the parabolic rate law. An activation energy of 25.6 kcal/mole was obtained for Co-10Nb, Co-20Nb, and Co-25Nb, while a value of 20.5 kcal/mole was found for Co-30Nb. All were two-phase alloys, consisting of solid solution α-Co and the intermetallic compound, NbCo3. The two-phase alloys formed a rather thick outer layer of cobalt sulfides and a heterophasic inner layer that was complex. The inner layer always contained the mixed sulfide CoNb2S4 which, depending on the alloy composition, coexisted with cobalt sulfide, NbS2, and / or NbCo3 particles. Short-time sulfidations showed that the solid solution initially sulfidized rapidly to form nodules of cobalt sulfide, whereas the NbCo3 phase formed a thin protective layer of NbS2. The nodules grew laterally until they coalesced into the continuous, outer thick layer, while the NbS2 completely or partially reacted with the cobalt sulfide to form CoNb2S4. Platinum markers were always found at the interface between the inner and outer scales, the location of the original metal surface.
Similar content being viewed by others
References
K. N. Strafford and J. R. Bird,J. Less Common Met. 68, 223 (1979).
S. Mrowec and K. Przybylski,High Temp. Mat. Processes 6, 1 (1984).
A. Davin,Cobalt 30, 19 (1966).
D. Coutsouradis and A. Davin, inHigh Temperature Metallic Corrosion by Sulfur and Its Compounds, by Z. A. Forolous, ed. (Electrochemical Society, New York, 1970), p. 132.
S. Mrowec, S. Rusiecki, and A. Wojtowicz,Bull. Pol. Acad. Chem. 34, 411 (1986).
D. P. Whittle, S. K. Verma, and J. Stringer,Corr. Sci. 13, 247 (1973).
S. K. Verma, D. P. Whittle, and J. Stringer,Oxid. Met. 5, 169 (1972).
T. Biegum, A. Bruckman, and S. Mrowec,Oxid. Met. 12, 157 (1978).
S. K. Verma, D. P. Whittle, and J. Stringer,Corr. Sci. 12, 545 (1972).
A. Davin, D. Coutsouradis, and L. Habraken,Werkst. Korr. 6, 517 (1971).
Metals Handbook, Metallography, Structures and Phases, Vol. 8, 8th ed. (American Society for Metals, Metals Park, Ohio (1973).
S. R. Shatynski,Oxid. Met. 11, 307 (1977).
F. Gesmundo, D. J. Young, and S. K. Roy,High Temp. Mat. Processes (to be published).
T. Rosenqvist,J. Iron Steel Inst. 176, 3 (1954).
D. L. Douglass,Corr. Sci. 8, 665 (1968).
K. Pryzybylski and D. Szwagierczak,Oxid. Met. 17, 267 (1982).
F. Gesmundo and F. Viani,Oxid. Met. 25, 269 (1986).
J. Stringer, inProceedings of the International Conference on Behavior of High Temperature Alloys in Aggressive Environments, I. Kirman, J. B. Mariott, M. Merz, P. R. Sahn, and D. P. Whittle, eds. (Metals Society, London 1980), p. 739.
F. Gesmundo, P. Nanni, and F. Viani, inProceedings of the Tenth International Symposium on the Reactivity of Solids, P. Barret and L. C. Dufour, eds. (Materials Science Monographs, Elsevier, New York, 1985), 28A, p. 175.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gleeson, B., Douglass, D.L. & Gesmundo, F. Effect of Nb on the high-temperature sulfidation behavior of cobalt. Oxid Met 31, 209–236 (1989). https://doi.org/10.1007/BF00846687
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00846687