Abstract
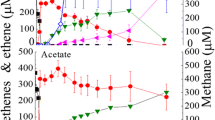
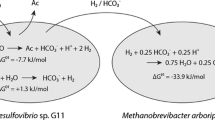
The butyrate-degradingSyntrophospora bryantii degrades butyrate and a propionate-degrading strain (MPOB) degrades propionate in coculture with the hydrogen- and formate-utilizingMethanospirillum hungatii orMethanobacterium formicicum. However, the substrates are not degraded in constructed cocultures with twoMethanobrevibacter arboriphilus strains which are only able to consume hydrogen. Pure cultures of the acetogenic bacteria form both hydrogen and formate during butyrate oxidation with pentenoate as electron acceptor and during propionate oxidation with fumarate as electron acceptor. Using the highest hydrogen and formate levels which can be reached by the acetogens and the lowest hydrogen and formate levels which can be maintained by the methanogens it appeared that the calculated formate diffusion rates are about 100 times higher than the calculated hydrogen diffusion rates.
Similar content being viewed by others
References
Amos DA & McInerney MJ (1990) Growth ofSyntrophomonas wolfei on short-chain unsaturated fatty acids. Arch. Microbiol. 154: 31–36
Beaty PS & McInerney MJ (1987) Growth ofSyntrophomonas wolfei in pure culture on crotonate. Arch. Microbiol. 147: 389–393
Bleicher K & Winter J (1994) Formate production and utilization by methanogens and by sewage sludge consortia — interference with the concept of interspecies formate transfer. Appl. Microbiol. Biotechnol. 40: 910–915
Boone DR & Bryant MP (1980) Propionate-degrading bacterium,Syntrophobacter wolinii sp. nov. gen. nov., from methanogenic ecosystems. Appl. Environ. Microbiol. 40: 626–632
Boone DR, Johnson RL & Liu Y (1989) Diffusion of the interspecies electron carriers H2 and formate in methanogenic ecosystems, and implications in the measurement of K M for H2 or formate uptake. Appl. Environ. Microbiol. 55: 1735–1741
Dong X, Cheng G & Stams AJM (1994a) Butyrate oxidation bySyntrophosporabryantii in coculture with different methanogens and in pure culture with pentenoate as electron acceptor. Appl. Microbiol. Biotechnol. 42: 647–652
Dong X, Plugge CM & Stams AJM (1994b) Anaerobic degradation of propionate by a mesophilic acetogenic bacterium in coculture and triculture with different methanogens. Appl. Environ. Microbiol. 60: 2834–2838
Dong X & Stams AJM (1995) Evidence for H2 and formate formation during syntrophic butyrate and propionate degradation. Anaerobe 1: 35–39
Dörner C (1992) Biochemie und Energetik der Wasserstoff-Freisetzung in der syntrophen Vergärung von Fettsäuren und Benzoat. Dissertation, University of Tübingen
Houwen FP, Dijkema C, Schoenmakers CHH, Stams AJM & Zehnder AJB (1987)13C-NMR study of propionate degradation by a methanogenic coculture. FEMS Microbiol. Lett. 41: 269–274
Houwen FP, Plokker J, Dijkema C & Stams AJM (1990) Enzymatic evidence for involvement of the methylmalonyl-CoA pathway in propionate oxidation bySyntrophobacter wolinii. Arch. Microbiol. 155: 52–55
Hungate RE (1967) Hydrogen as an intermediate in the rumen fermentation. Arch. Microbiol. 59: 158–164
Koch M, Dolfing J, Wuhrmann K & Zehnder AJB (1983) Pathway of propionate degradation by enriched methanogenic cultures. Appl. Environ. Microbiol. 45: 1411–1414
McInerney MJ, Bryant MP, Hespell RB & Costerton JW (1981)Syntrophomonas wolfei gen. nov. sp. nov., an anaerobic, syntrophic, fatty acid-oxidizing bacterium. Appl. Environ. Microbiol. 41: 1029–1039
Plugge CM, Dijkema C & Stams AJM (1993) Acetyl-CoA cleavage pathway in a syntrophic propionate oxidizing bacterium growing on fumarate in the absence of methanogens. FEMS Microbiol. Lett. 110: 71–76
Roy F, Samain E, Dubourguier HC & Albagnac G (1986)Syntrophomonas sapovorans sp. nov. a new obligately proton reducting anaerobe oxidizing saturated and unsaturated long chain fatty acids. Arch. Microbiol. 145: 142–147
Schauer NL, Brown DP & Ferry JG (1982) Kinetics of formate metabolism inMethanobacterium formicicum andMethanospirillum hungatei. Appl. Environ. Microbiol. 44: 540–554
Schink B (1992) Syntrophism among prokaryotes. In Balows A, Trüper HG, Dworkin M, Harder W & Schleifer K-H (Eds) The Prokaryotes 2nd ed. (pp 276–299). Springer Verlag, New York
Seitz HJ, Schink B & Conrad R (1988) Thermodynamics of hydrogen metabolism in methanogenic cocultures degrading ethanol or lactate. FEMS Microbiol. Lett. 55: 119–124
Shelton DR & Tiedje JM (1984) Isolation and partial characterisation of bacteria in an anaerobic consortium that mineralizes 3-chlorobenzoic acid. Appl. Environ. Microbiol. 48: 840–848
Stams AJM (1994) Metabolic interactions between anaerobic bacteria in methanogenic environments. Antonie van Leeuwenhoek 66: 271–294
Stams AJM, van Dijk J, Dijkema C & Plugge CM (1993) Growth of syntrophic propionate-oxidizing bacteria with fumarate in the absence of methanogenic bacteria. Appl. Environ. Microbiol. 59: 1114–1119
Stieb M & Schink B (1985) Anaerobic oxidation of fatty acids byClostridium bryantii sp. nov., a sporeforming, obligately syntrophic bacterium. Arch. Microbiol. 140: 387–390
Thiele JH & Zeikus JG (1988) Control of interspecies electron flow during anaerobic digestion: significance of formate transfer versus hydrogen transfer during syntrophic methanogensis in flocs. Appl. Environ. Microbiol. 54: 20–29
Van Kuijk BLM & Stams AJM (1995) Sulfate reduction by a syntrophic propionate oxidizing bacterium. Proceedings of the workshop “Anaerobic processes for bioenergy and environment”. Copenhagen 25–27 January 1995.
Wallrabenstein C, Hauschild E & Schink B (1994) Pure culture and cytological properties of ‘Syntrophobacter wolinii’. FEMS Microbiol. Lett. 123: 249–254
Wofford NQ, Beaty PS & McInerney MJ (1986) Preparation of cellfree extracts and the enzymes involved in fatty acid metabolism inSyntrophomonas wolfei. J. Bacteriol. 167: 179–185
Wolin MJ (1982) Hydrogen transfer in microbial communities. In: Bull AT & Slater JH (Eds) Microbioal interactions and communities (pp 323–356). Academic Press, London
Wu W-M, Rickley RF, Jain MK & Zeikus JG (1993) Energetics and regulations of formate and hydrogen metabolism byMethanobacterium formicicum. Arch. Microbiol. 159: 57–65
Zhao H, Yang D, Woese CR & Bryant MP (1990) Assignment ofClostridium bryantii toSyntrophosporabryantii gen. nov., comb. nov. on the basis of a 16S rRNA sequence analysis of its crotonategrown pure culture. Int. J. Syst. Bacteriol. 40: 40–44
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stams, A.J.M., Dong, X. Role of formate and hydrogen in the degradation of propionate and butyrate by defined suspended cocultures of acetogenic and methanogenic bacteria. Antonie van Leeuwenhoek 68, 281–284 (1995). https://doi.org/10.1007/BF00874137
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00874137