Abstract
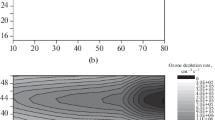
Starting with the average actual distribution of ozone (Dütsch [15]) and temperature in the stratosphere, we have calculated the solar intensity as a function of wavelength and the instantaneous rates (molecules cm−3 sec−1) for each Chapman reaction and for each of several reactions of the oxides of nitrogen. The calculation is similar to that ofBrewer andWilson [5]. These reaction rates were calculated independently in each volume element in spherical polar coordinates defined by ΔR=1 km from zero to 50, δϑ=5° latitude, and δø=15° longitude (thus including day and night conditions). Calculations were made for two times: summer-winter (January 15) and spring-fall (March 22). As input data we take observed solar intensities (Ackerman [1]) and observed, critically evaluated. constants for elementary chemical and photochemical reactions; no adjustable parameters are employed. (These are not ‘photochemical equilibrium’ calculations.) According to the Chapman model, the instantaneous, integrated, world-wide rate of formation of ozone from sunlight is about five times faster than the rate of ozone destruction, and locally (lower tropical stratosphere) the rate of ozone formation exceeds the rate of destruction by a factors as great as 1000. The global rates of increase of ozone are more than 50 times faster thanBrewer andWilson's [5] estimate of the average annual transfer rate of ozone to the troposphere. The rate constants of the Chapman reactions are believed to be well-enough known that it is highly improbable that these discrepancies are, due to erroneous rate constants. It is concluded that something else besides neutral oxygen species is very important in stratospheric ozone photochemistry. The inclusion of a uniform concentration of the oxides of nitrogen (NOx as, NO and NO2) averaging 6.6×10−9 mole fraction gives a balance between global ozone formation and destruction rates. The inclusion of a uniform mole fraction of NOx at 28×10−9 also gives a global balance. These calculations support the hypethesis (Crutzen [10],Johnston [24]) that the oxides of nitrogen are the most important factor in the global, natural ozone balance. Several authors have recently evaluated the natural source strength of NOx in the stratosphere; the projected fleets of supersonic transports would constitute an artificial source of NOx about equal to the natural value, thus promising more or less to double an active natural stratospheric ingredient.
Similar content being viewed by others
References
M. Ackerman,Ultraviolet Solar Radiation Related to Mesospheric Processes. Mesospheric Models and Related Experiments, edited byG. Fiocco (D. Reidel Publishing Company, Dordrecht, Holland 1971), pp. 149–159.
Australian Academy of Sciences Report No. 15,Atmospheric Effects of Supersonic Aircraft (1972).
D. R. Bates andP. B. Hayes,Atmospheric nitrous, oxides, Planet. Space Sci.15 (1967), 189–197.
D. L. Baulch., D. D. Drysdale andD. G. Horne,Critical Evaluation of Rate Data for Homogeneous Gas Phase Reactions, Vol. 5. (The University, Leeds, England 1970).
A. W. Brewer andA. W. Wilson,The regions of formation of atmospheric ozone, Roy. Meteorol. Soc. Quart. J.94 (1968), 249–265.
R. T. Brinkman, A. E. S. Green andC. A. Barth, 1966.A Digitalized Solar Ultraviolet Spectrum, NASA Technical Report No.32-951. Jet Propulsion Laboratory, Pasadena, California.
S. Chapman,A theory of upper-atmosphere ozone Mem. Roy. Meteorol. Soc.3 (1930), 103.
Climatic Impact Assessment Program, research program by the Department of Transportation, 1972–1974.
H. L. Crutcher,Temperature and Humidity in the Troposphere. World Survey of Climatology, Vol. 4, edited byD. F. Rex (Elsevier Publishing Company, Amsterdam-London-New York, 1969), pp. 45–83. b.Handbook of Geophysics (1960).
P. J. Crutzen,The influence of nitrogen oxides on the atmospheric ozone, content, Quarterly J. Roy. Meteorol. Soc.96 (1970), 320–325.
P. J. Crutzen,Ozone production rates in an oxygen-hydrogen-nitrogen atmosphere, J. Geophys. Res.76 (1971), 7311–7327.
R. E. Huie, J. T. Herron andD. D. Davis,Absolute rate constants for the reaction O+O 2+M→O3+M over the temperature range 200–346 K. J. Phys. Chem.76 (1972), 2653–2658.
G. B. M. Dobson,Exploring the Atmosphere (The Clarendon Press, Oxford 1968), p. 126.
H. U. Dütsch, Chemical reactions in the lower and upper atmosphere, International Symposium, Stanford Research Institute, 1961.
H. U. Dütsch,Atmospheric Ozone and Ultraviolet Radiation, World Survey of Climatology, Vol. 4, edited byD. F. Rex (Elsevier Publishing Company, Amsterdam, London, New York 1969), pp. 383–432
R. Graham, Measurements in this laboratory, 1972.
B. G. Hunt,The need for a modified photochemical theory of the ozonosphere, J. Atmospheric Sci.23 (1966), 88–95.
B. G. Hunt,Photochemistry of ozone in a moist atmosphere, J. Geophys. Res.71 (1966), 1385–1398.
F. S. Johnson, J. D. Purcell andR. Tousey,Studies of the Ozone Layer Over New Mexico, Rocket Exploration of the Upper Atmosphere (Pergamon Press, London 1954), pp. 189–199.
H. S. Johnston andD. M. Yost,The kinetics of the rapid gas reaction between ozone and nitrogen dioxide, J. Chem. Phys.17 (1949), 386–392.
H. S. Johnston, L. Foering andR. J. Thompson,Kinetics of the thermal decomposition of nitric acid vapor. II. Mechanism, J. Phys. Chem.57 (1953), 390–395.
H. S. Johnston andH. J. Crosby,Kinetics of the fast reaction between ozone and nitric oxide. J. Chem. Phys.22 (1954), 689–692.
H. S. Johnston,Kinetics of Neutral Oxygen Species, U.S. Department of Commerce, National Standard Reference Data System, National Bureau of Standards Vol. 20 (1954).
H. S. Johnston,Reduction of stratospheric ozone by nitrogen oxide catalysts from supersonic transport exhaust,Science 173 (1971), 517–522.
The same material in a much fuller form, Lawrence Radiation Laboratory Report UCRL-20568 TID-4500.
F. Kaufman andJ. L. McCrumb,Kinetics of the O+O 3 reaction,. J. Chem. Phys.57 (1972), 1270–1276.
K. R. Langley andW. D. McGrath,The ultraviolet photolysis of ozone in the presence of water vapour. Planet. Space Sci.19 (1971), 413–418.
P. A. Leighton,Photochemistry of Air Pollution (Academic Press, New York and London 1961).
M. B. McElroy andJ. C. McConnell,Nitrous oxide: a natural source of stratospheric NO. J. Atmosph. Sci.28 (1971), 1095–1098.
M. Nicolet andE. Vergison,L'Oxyde azoteux, dans la stratosphere. Aeronom. Acta90 (1971), 1–16.
H. K. Paetzold,Vertical Atmospheric Ozone Distribution, Ozone Chemistry and Technology, Advances in Chemistry No. 21 (American Chemical Society1959), pp. 209–220.
M. Sissenwine,Standard and Supplemental Atmospheres inWorld Survey of Climatology Vol. 4, edited byD. F. Rex (Elsevier Publishing Company, Amsterdam, London, New York 1969), pp. 5–44.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Johnston, H., Whitten, G. Instantaneous photochemical rates in the global stratosphere. PAGEOPH 106, 1468–1489 (1973). https://doi.org/10.1007/BF00881099
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00881099