Abstract
Mo2Cl4 Pic 4·CHCl3 (A) (Pic=4-methylpyridine) and Mo2Br4 Pic 4 (B) crystallize in the monoclinic space group.A inC2/c (No. 15) witha=15.175 (4),b=10.847 (2),c=19.946 (6) Å and β=104.52 (2)°;D o=1.71 (2),D c =1.72 gcm−3 forZ=4.B inP2l/n (No. 14) witha=9.270 (3),b=16.614 (5),c=9.305 (3) Å and β=91.96 (5)°;D o=2.03 (3),D c =2.05 gcm−3 forZ=2.
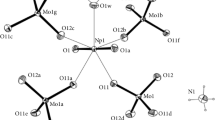
Two halogens and 4-methylpyridines of the MoX 2 Pic 2 group are in the trans position. Mo−Mo bond lengths are 2.153 96) forA and 2.150 92) Å forB. Both molecules are situated on the inversion center resulting in the eclipsed configuration of the ligands around the molybdenum pair. The structure ofB has been refined to the conventionalR factors of 0.08 and 0.098. Disorder on the part of 4-methylpyridines and chloroform molecules stopped the refinement ofA at the endR value of 0.175.
Mean Mo−X and Mo−N bonding distances are 2.40 (2), 2.25 (5) Å forA and 2.53 (3), 2.25 (1) Å forB.
Similar content being viewed by others
Literatur
J. San Filippo, jr., H. J. Sniadoch undR. L. Grayson, Inorg. Chem.13, 2121 (1974).
J. V. Brenčič, D. Dobčnik undP. Šegedin, Mh. Chem.107, 395 (1976).
T. Nimry undR. A. Walton, Inorg. Chem.17, 510 (1978).
J. San Filippo, jr., Inorg. Chem.11, 3140 (1972).
F. A. Cotton, B. A. Frenz, J. R. Ebner undR. A. Walton, Inorg. Chem.15, 1630 (1976).
F. A. Best, T. J. Smith undR. A. Walton, Inorg. Chem.17, 99 (1978).
N. B. Colthup, L. H. Daly undS. E. Wiberly, Introduction to Infrared and Raman Spectroscopy, S. 411. New York: Academic Press. 1964.
J. V. Brenčič undP. Šegedin, Z. anorg. allg. Chem.423, 266 (1976).
G. Germain, P. Main undM. M. Woolfson, Acta Crystallogr.A 27, 368 (1971).
D. T. Cromer undJ. T. Waber, Acta Crystallogr.18, 104 (1965).
D. T. Cromer, Acta Crystallogr.18, 17 (1965).
The X-Ray System (J. M. Stewart, Hrsg.). Computer Science Center, University Maryland (1970).
F. A. Cotton, Chem. Soc. Rev.4, 27 (1975).
J. V. Brenčič undF. A. Cotton, Inorg. Chem.9, 346 (1970).
M. J. Bennet, J. V. Brenčič undF. A. Cotton, Inorg. Chem.8, 1060 (1969).
F. A. Cotton undB. J. Kalbacher, Inorg. Chem.15, 522 (1976).
R. Saillant, R. B. Jackson, W. E. Streib, K. Folting undR. A. D. Wentworth, Inorg. Chem.403, 218 (1974).
J. V. Brenčič, Z. anorg. allg. Chem.403, 218 (1974).
A. Mawby undG. E. Pringle, J. Inorg. Nucl. Chem.34, 517 (1972).
M. G. B. Drew undJ. D. Wilkins, J. Chem. Soc. Dalton1973, 2664.
D. Babel, J. Solid State Chem.4, 410 (1972).
J. V. Brenčič, I. Leban undP. Šegedin, Z. anorg. allg. Chem.427, 85 (1976).
J. A. Potenza, R. J. Johnson undJ. San Filippo, jr., Inorg. Chem.15, 2215 (1976).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brenčič, J.V., Golič, L., Leban, I. et al. Komplexe des zweiwertigen Molybdäns, Derivate des Oktahalodimolybdat(II)-Ions, 4. Mitt.: Darstellungen und Kristallstrukturen von zwei neutralen Komplexen mit 4-Methylpyridin. Monatshefte für Chemie 110, 1221–1228 (1979). https://doi.org/10.1007/BF00910969
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00910969