Summary
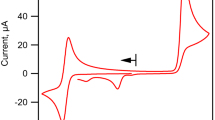
Several carboxy-substituted ferrocene compounds are prepared and investigated by cyclic voltammetry in acetonitrile solution. The half-wave potentials of most of the acids studied (E 1/2=0.34−0.58 V versus s.c.e.) are more positive than that of ferrocene (0.33 V), reflecting a diminished susceptibility to oxidation of these compounds relative to the parent metallocene. Only β-ferrocenylpropionic acid (0.325 V) is effectively identical with the latter in its oxidation behaviour, and γ-ferrocenylbutyric acid (0.31 V) tends to be more readily oxidized. The results are of interest for subsequent chemical oxidation studies of ferrocenylcarboxylic acids.
Similar content being viewed by others
References
P. C. Reeves,Org. Synth,56, 28 (1977).
D. Lednicer, J. K. Lindsay and C. R. Hauser,J. Org. Chem.,23, 653 (1958).
A. Dormond and J. Décombe,Bull. Soc. Chim. Fr., 3673, (1968).
K. L. Rinehart, R. J. Curby, and P. E. Sokol,J. Am. Chem. Soc.,79, 3420 (1957).
A. Ratajczak, B. Misterkiewicz,J. Organometal. Chem.,179, 181 (1979).
In ferrocenoic acid, in which the conjugated α-carbonyl group is coplanar with the adjacent cyclopentadienyl ring, the molecular geometry favours intermolecular hydrogen bonding; a dimer structure in the crystal was indeed established by x-ray diffraction: F. A. Cotton and A. H. Reid,Acta Crystallogr,C41, 686 (1985). Consistent with an intermolecular bonding mode in the solid state, the band maximum appears at 1712 cm−1, i.e., at the position expected for non-bonded carboxyl, when the spectrum of (2) is recorded in (0.02m) diethyl ether solution.
The geometry in(3)–(8), while allowing for a minor extent of hydrogen bonding to Fe, would be expected to favour hydrogen bond formation through ‘back-biting’ interaction with the π-electron system of the (substituted) cyclopentadienyl ring. Both types of intramolecular bonding are known to operate, e.g., in 2-ferrocenylethanols(8). However, the solution spectra (diethyl ether, 0.02m) of these acids all display ν(CO) as a sharp and strong band at 1715–1710 cm−1, with but negligibly weak absorption shown near 1630 cm−1. This suggests intramolecular bonding, if operative in these compounds, to be weak enough to be broken on dissolution.
D. S. Trifan and R. Bacskai,J. Am. Chem. Soc.,82, 5010 (1960). M. J. Nugent and J. H. Richards,J. Am. Chem. Soc.,91, 6138 (1969). F. H. Hon and T. T. Tidwell,J. Org. Chem.,37, 1782 (1972). A. W. Baker and D. E. Bublitz,Spectrochim. Acta,22, 1787 (1966).
R. S. Nicholson,Anal. Chem.,36, 1406 (1966); adapted for positive direction of potential scan in forward mode.
R. S. Nicholson,Anal. Chem.,37, 1351 (1965).
P. T. Kissinger and W. R. Heineman,J. Chem. Educ.,60, 702 (1983).
Measured at a point 85.17% of anodic peak(9).
R. N. Adams,Electrochemistry at Solid Electrodes, Marcel Dekker, New York, 1969, p. 148.
D. H. Evans, K. M. O'Connell, R. A. Petersen, and M. J. Kelly,J. Chem. Educ.,60, 290 (1983).
E. Heitz and G. Kreysa,Grundlagen der Technischen Elektrochemie, 2nd Edit, Verlag Chemie, Weinheim, 1980, p. 10.
H. Hennig and O. Gürtler,J. Organometal. Chem.,11, 307 (1968).
R. Herrmann, A. J. L. Pombeiro, M. E. N. P. Rodrigues, and I. Ugi,Port. Electrochim. Acta,2, 57 (1984).
J. E. Gorton, H. L. Lentzner, and W. E. Watts,Tetrahedron 27, 4353 (1971), and refs. cited therein.
Y. S. Sohn, D. N. Hendrickson, and H. B. Gray,J. Am. Chem. Soc.,93, 3603 (1971).
Extending the scope of this work, we included a number of other ferrocene derivatives, such as ferrocenylcarbinol, methyl- and 1, 1′-dimethylferrocene, and 1, 1′-diacetylferrocene. The observed λmax values of band system IV ranged from 270 to 275 nm i.e., well within the limits given for compounds(1)–(8).
In ferrocene derivatives bearing substituents conjugated with the attached cyclopentadienyl ring(s), for example, π* ← π transitions contribute significantly to a powerful absorption appearing at 310–350 nm superimposed on the ligandfield band (system III) in that region. The spectral pattern may be further complicated by (weaker) π* ← n absorption emerging at λ>300 nm in the spectra of α-carbonylsubstituted ferroceness.
K. L. Rinehart, K. L. Motz, and S. Moon,J. Am. Chem. Soc.,79, 2749 (1957).
R. L. Schaaf,J. Org. Chem.,27, 107 (1962).
V. Weinmayr,J. Am. Chem. Soc.,77, 3009 (1955).
J. M. Osgerby and P. L. Pauson,J. Chem. Soc., 656 (1958).
D. Lednicer, T. A. Mashburn, and C. R. Hauser,Org. Synth.,40, 52 (1960).
S. Sahami and M. J. Weaver,J. Solution Chem.,10, 199 (1981).
W. F. Little, C. N. Reilley, J. D. Johnson, and A. P. Sanders,J. Am. Chem. Soc.,86, 1382 (1964).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Blom, N.F., Neuse, E.W. & Thomas, H.G. Electrochemical characterization of some ferrocenylcarboxylic acids. Transition Met Chem 12, 301–306 (1987). https://doi.org/10.1007/BF01024018
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01024018