Abstract
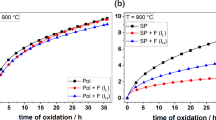
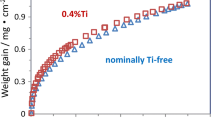
Alumina-forming ODS superalloys are excellent oxidation-resistant materials. Their resistance relies upon the establishment of a stable, slow-growing, and adherent α-alumina. In the present investigation, these alloys exhibited unstable and relatively less adherent θ-alumina phase, which increased the oxidation rate in the transient stage and converted into α-alumina in the later part of the exposure. The oxide-growth process was found to depend upon various parameters such as temperature, time, and presence of an active elecment in the superalloy. Characterization carried out by XRD, SEM/EDAX, and AES on oxidized ODS and non-ODS alloys demonstrated a significant influence of the active element, Y, on the transformation of θ- to α-alumina. SIMS analysis of two-stage oxidation at 900°C for two different durations evidently showed that the change in the transport process is due to θ-to-α-alumina transformation. On the basis of these results, a new and consistent mechanism is proposed to explain the influence of θ-alumina and its transformation on growth kinetics and the effect of yttrium on the transformation leading to good scale adherence and oxidation resistance.
Similar content being viewed by others

References
W. J. Quadakkers, H. Holzbrecher, K. G. Briefs, and H. Beske,Oxid. Met. 32, 67 (1989).
W. J. Quadakkers,Werks. Korros. 41, 659 (1990).
D. P. Moon,Mater. Sci. Technol. 5, 754 (1989).
J. Doychak, Master thesis, Case Western Reserve University, Cleveland, Ohio, 1984.
W. J. Quadakkers, K. Schmidt, H. Grubmeier, and E. Wallura,Mater. High Tem. 10, 23 (1992).
P. T. Mosely, K. R. Hyde, B. A. Bellany, and G. Tappin,Corros. Sci. 24, 547 (1984).
G. C. Rybicki and J. Smialek,Oxid. Met. 31, 275 (1989).
J. Dokychak, J. L. Smialek, and T. E. Mitchell,Metall. Trans. 20A, 499 (1989).
M. W. Brumm and H. J. Grabke,Corros. Sci. 33, 1677 (1992).
P. A. Van Manen, E. W. A. Young, D. Schalkord, C. J. Vander wekken, and J. H. W. de Wit,Surf. Interface Anal. 12, 391 (1987).
J. Doychak and M. Ruhle,Oxid. Met. 32, 431 (1989).
B. A. Pint, J. R. Martin, and L. Hobbs, unpublished research.
B. A. Pint, A. Jain and L. W. Hobbs,MRS Symp. Proc. 213, 981 (1991).
R. Prescott and M. J. Graham,Oxid. Met. 38, 233 (1992).
M. J. Graham, J. I. Eldridge, D. F. Mitchell, and R. J. Hussey,Mater. Sci. Forum. 43, 207 (1989).
J. Jedlinsky and R. Borchardt,Oxid. Met. 36, 318 (1991).
J. Jedlinsky,Oxid. Met. 39, 55 (1993).
R. A. Versaci, D. Clemens, W. J. Quadakkers, and R. Hussey,Solid State Ionics,59, 235 (1993).
K. Przybylski, A. J. Garratt-Reed, and G. J. Yurek,J. Electrochem. Soc. 135, 509 (1988).
P. Burtin, J. P. Brunelle, and M. Sonstelle,Appl. Catal. 34, 239 (1987).
T. A. Ramanarayanan, M. Raghavan, and R. Petkovic-Luton,J. Electrochem. Soc. 131, 923 (1984).
J. Jedlinsky and G. Borchardt,Proc. Symp. on Oxide Films on Metals and Alloys (The Electrochemical Society, Inc., 1992), pp. 67–76.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Prasanna, K.M.N., Khanna, A.S., Chandra, R. et al. Effect ofθ-alumina formation on the growth kinetics of alumina-forming superalloys. Oxid Met 46, 465–480 (1996). https://doi.org/10.1007/BF01048641
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01048641