Abstract
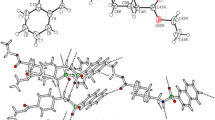
The crystal and molecular structures of the 1∶1 complexes sulfadimidine·2-aminobenzoic acid (I) and sulfadimidine·4-aminobenzoic acid (II) are described and compared with known structures containing sulfadimidine. In each complex, molecular association is maintained by one N-H⋯O hydrogen bond, and one O-H⋯N hydrogen bond involving the imino N and one pyrimidinyl N atom of sulfadimidine and the two carboxyl O atoms of the aromatic acid. The conformation of the sulfadimidine molecule inII is unusual for anN 1-substituted arylsulfonamide. Molecular dimensions for the complexed aminobenzoic acids are virtually the same as those reported for the free acids. Partial correlation between carboxylic acid strengths and the hydrogen bonded N⋯O distances is observed in sulfadimidine complexes of this type.
Similar content being viewed by others
References
Basak, A. K., Mazumdar, S. K., and Chaudhuri, S. (1983).Acta Crystallogr. C 39, 492–494.
CRC Handbook of Chemistry and Physics (1985–1986). R. C. Weast (ed.) (CRC Press, Cleveland, Ohio).
Ghose, S., Chakrabarti, C., and Dattagupta, J. K. (1987).J. Crystallogr. Spectrosc. Res. 17, 197–206.
Ghose, S., Chakrabarti, C., Dattagupta, J. K., Le Page, Y., and Trotter, J. (1988).Acta Crystallogr. C 44, 1810–1813.
Ghosh, M., Basak, A. K., Mazumdar, S. K., and Sheldrick, B. (1989).J. Crystallogr. Spectrosc. Res. 19, 289–298.
Grahame-Smith, D. G., and Aronson, J. K. (1985). InThe Oxford textbook of clinical pharmacology and drug therapy. (Oxford University Press, Oxford OX2 6DP), p. 755.
International Tables for X-Ray Crystallography (1974). Vol. IV. (Kynoch Press, Birmingham) (Present distributor: Reidel, D., Dordrecht).
Kálmán, A., Czugler, M., and Argay, G. (1981).Acta Crystallogr. B 37, 868–877.
Lai, T. F., and Marsh, R. E. (1967).Acta Crystallogr. B 22, 885–893.
Merck Index, 11th Edition (1989). 434. S. Budavari (ed.) (Merck & Co., Inc. Rahway, N. J.).
Motherwell, S. (1979).Pluto,A Program for plotting molecular and crystal structures (University of Cambridge, England).
Nagai, K., Takayama, K., and Nanbu, N. (1979). (Yamanouchi Pharmaceutical Co., Ltd.) Jpn. Kokai Tokkyo Koho, Patent 79 16,494.
Nakahara, W., Inukai, F., Ugami, S., and Nagata, Y. (1945).Sci. Papers Inst. Phys. Chem. Res. 39, 39–44.
Nardelli, M. (1983).Comput. Chem. 7(3), 95–98.
North, A. C. T., Philips, D. C., and Scott Mathews, F. (1968).Acta Ctystallogr. A 24, 351–359.
Papastephanou, C., and Frantz, M. (1978). InAnalytical Profiles of Drug Substances, Vol. 7 K. Florey. (ed.) (Academic Press, New York), p. 414.
Patel, U., Haridas, M., and Singh, T. P. (1988).Acta Crystallogr. C 44, 1264–1267.
Rambaud, J., Maury, L., Pauvert, B., Audran, M., Lasserre, Y., Berge, G., and Declercq, J. P. (1985).Acta Crystallogr. C 41, 133–134.
Shefter, E., and Sackman, P. (1971).J. Pharm. Sci. 60, 282–286.
Sheldrick, G. M. (1976).Shelx,A System of Computer Programs for X-Ray Structure Determination (University of Cambridge, England).
Simonov, Y., Battaglia, L. P., Corradi, A. B., Ianelli, S., Pelosi, G., Ganin, E., and Lukjanenko, N. (1990).J. Incl. Phenom. 9, 181–194.
Structure Determination Package (1982). B. A. Frenz and Associates Inc., College Station, Texas 77840.
Takazawa, H., Ohba, S., and Saito, Y. (1986).Acta Crystallogr. C 42, 1880–1881.
Tiwari, R. K., Haridas, M., and Singh, T. P. (1984).Acta Crystallogr. C 40, 655–657.
Williams, R. A. D., and Kruk, Z. L. (1981). InThe Biochemistry and Pharmacology of Antibacterial Agents. (Croom Helm biology in medicine series, London), pp. 24–28.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Caira, M.R. Molecular complexes of sulfonamides. Part 1. 1∶1 complexes between sulfadimidine [4-amino-N-(4,6-dimethyl-2-pyrimidinyl)benzenesulfonamide] and 2- and 4-aminobenzoic acids. Journal of Crystallographic and Spectroscopic Research 21, 641–648 (1991). https://doi.org/10.1007/BF01161088
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01161088