Summary
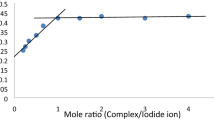
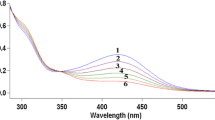
Kinetic studies in water-ethanol solution on the replacement of the aqua-ligands in [Cr(H2O)5(OH)]2+ by 2,2′-bipyridine (bipy) and 1, 10-phenanthroline (phen) have been made spectrophotometrically. In both the cases the reaction proceeds by a path which is first order with respect to the base. The reaction is catalysed by NO −3 and the rate increases with the increase of pH. We propose the rate law
where k1 is the water dissociation rate of the substrate complex, k−1 is the aquation and k2 is the ligand capturing rate of the pentacoordinate intermediate [Cr(H2O)4(OH)]2+. Experimental results are consistent with a dissociative mechanism. Variation of dielectric constant of the medium was used to verify the mechanistic conclusion in the reaction with bipy.
Similar content being viewed by others
References
D. Banerjea and C. Chatterjee,J. Inorg. Nucl. Chem.,31, 3845 (1969).
D. Banerjea and S. Dutta Chaudhury,J. Inorg. Nucl. Chem.,30, 871 (1968).
R. E. Hamm, R. L. Johnson, R. H. Perkins and R. E. Davis,J. Am. Chem. Soc.,80, 4469 (1985).
F. R. Hartley,Aust. J. Chem.,21, 2723 (1968).
M. Bhattacharya and G. S. De,Indian J. Chem.,20A, 780 (1981).
J. N. Mondal and G. S. De,Indian J. Chem.,19A, 25 (1980).
D. Banerjea and S. Sarkar,Z. Anorg. Allg. Chem.,393, 301 (1972).
J. H. Espenson,Inorg. Chem.,8, 1554 (1969).
D. Thusius,Inorg. Chem.,10, 1106 (1971).
B. K. Niogy and G. S. De,Proc. Indian Acad. Sci. (Chem. Sci.),92, 153 (1983).
B. K. Niogy and G. S. De,Indian J. Chem.,24A, 208 (1985).
H. G. Mitra Mustofy and G. S. De,J. Indian Chem. Soc.,63, 1040 (1986).
H. G. Mitra Mustofy and G. S. De,Proc. Indian Acad. Sci. (Chem. Sci.),98, 255 (1987).
P. Moor and F. Basolo,Inorg. Chem.,4, 1671 (1965).
S. Glasstone,Text Book of Physical Chemistry, MacMillan, 1977, p. 1116.
P. M. Brown and G. M. Harris,Inorg. Chem.,3, 1334 (1964).
M. C. Ghosh and P. Banerjee,J. Chem. Research(S), p. 271 (1984).
J. P. Hunt and R. A. Plane,J. Am. Chem. Soc.,76, 5960 (1954).
V. V. Udovenko, L. R. Reiter and E. P. Shkurman,Russ. J. Inorg. Chem.,6, 838 (1973).
M. N. Bishnu, B. Chakraborty, R. N. Banerjee and D. Banerjea,J. Coord. Chem.,13, 63 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mustofy, H.G.M., De, G.S. Mechanistic studies on the reactions of pentaaquahydroxochromium(III) ion with 2,2′-bipyridine and 1, 10-phenanthroline. Transition Met Chem 13, 196–200 (1988). https://doi.org/10.1007/BF01171324
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01171324