Summary
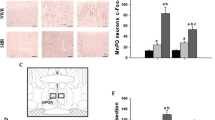
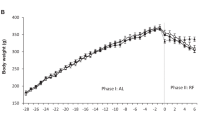
Male Wistar rats were housed in running wheel cages and were restricted in their food intake, in order to reduce the initial body weight by 30% within 10 days. Rats increased their daily running up to distances between 7 and 11 km compared to the maximum 2.5 km in controls fed ad libitum. The hypothalamic noradrenaline (NE) turnover, as estimated by the concentration of the major metabolite MHPG, was significantly decreased in semistarved sedentary rats compared to controls. Hyperactivity resulted in marked elevation of NE turnover at all time points examined. Semistarvation-induced decreases of dopamine (DA) turnover as estimated by the concentrations of its major metabolite DOPAC, could also be compensated by hyperactivity. The circadian pattern of NE turnover parallels the pattern of running activity. MHPG levels at times of high activity were even higher than in controls fed ad libitum (p<0.01). The availability of the precursor tyrosine, as indicated by the ratio of plasma tyrosine to the large neutral amino acids, was significantly decreased in semistarvation (p<0.0001); hyperactivity caused a further decrease (p<0.001), indicating that tyrosine availability is not, under these conditions, a limiting factor for noradrenaline turnover. The combined influence of semistarvation and hyperactivity on central catecholamine turnover in the rat is discussed as an animal model for the effects of malnutrition and heavy exercise often observed in anorexia nervosa.
Similar content being viewed by others
References
Belova TI, Jonsson G (1982) Blood brain barrier permeability and immobilization stress. Acta Physiol Scand 116: 21–29
Chang JY, Knecht R, Braun DG (1983) Amino acid analysis in the picomole range by precolumn derivatization and high-performance liquid chromatography. Methods Enzymol 91: 41–48
Elam M, Svensson TH, Thoren P (1987) Brain monoamine metabolism is altered in rats following spontaneous, long-distance running. Acta Physiol Scand 130: 313–316
Hudson Ph, Knott PJ, Curton G (1980) Effect of isoprenaline infusion on the distribution of tryptophan, tyrosine, and isoleucine, between brain and other tissues. Biochem Pharmacol 29: 509–516
Kanarek RB, Collier GH (1983) Self-starvation: a problem of overriding the satiety signal? Physiol Behav 30: 307–311
Kaye WD, Ebert MH, Raleigh M, Lake CR (1984) Abnormalities in CNS monoamine metabolism in anorexia nervosa. Arch Gen Psychiatry 41: 350–355
Kennett GA, Curzon G, Hunt A, Patel AJ (1986) Immobilization decreases amino acid concentrations in plasma but maintains or increases them in brain. J Neurochem 46: 208–212
Korf J, Aghajanian GK, Roth RH (1973) Stimulation and destruction of the locus coeruleus: opposite effects on 3-hethoxy-4-hydroxyphenylglycol-sulfate levels in the rat cerebral corts. Eur J Pharm 21: 305–310
Kron L, Katz JL, Curzyuski G, Weiner H (1978) Hyperactivity in anorexia nervosa: a fundamental clinical feature. Compr Psych 19: 433–440
Laessle RG, Schweiger U, Pirke KM (1988) Mood and orthostatic norepinephrine response in anorexia nervosa. Psychiatry Res 24: 87–94
Landsberg L, Young JB (1978) Fasting, feeding and regulation of the sympathetic nervous system. New Engl J Med 298: 1295–1301
Levitsky DA (1974) Feeding conditions and intermeal relationships. Physiol Behav 12: 779–787
Milner JD, Wurtman RJ (1986) Catecholamine synthesis: physiological coupling to precursor supply. Biochem Pharmacol 35: 875–881
Nudel DB, Norman G, Nussbaum MP, Shenker IR (1984) Altered exercise performance and abnormal sympathetic responses to exercise in patients with anorexia nervosa. J Pediatr 105: 34–37
Pardridge WM, Odendorff WH (1975) Kinetic analysis of blood-brain barrier transport of amino. acids. Biochim Biophys Acta 401: 128–136
Pirke KM, Spyra B, Warnhoff M, Kuederling I, Dorsch G, Gramsch C (1984) Effect of starvation on central neurotransmitter systems and on endocrine regulation. In: Pirke KM, Ploog D (eds) The psychobiology of anorexia nervosa. Springer, Berlin Heidelberg New York Tokyo
Pirke JM, Eckert M, Ofers B, Goebel G, Spyra B, Schweiger U, Tuschl RJ, Fichter MM (1989) Plasma norepinephrine response to exercise in bulimia, anorexia nervosa and controls. Biol Psychiatry 25: 799–802
Potter CD, Borer KT (1983) Opiate-receptor blockade reduces voluntary running but not self-stimulation in hamsters. Pharmacol Biochem Behav 18: 217–233
Richter CPA (1922) A behavioristic study of the activity of the rat. Comp Psychol Monogr 1: 1–55
Routtenberg A, Kuznesoff AW (1967) Self-starvation of rats living in activity wheels on a restricted feeding schedule. J Comp Physiol Psychol 64: 414–421
Russell JL, Epling WF, Pierce D, Amy RM, Boer DP (1967) Induction of voluntary prolonged running by rats. J Appl Physiol 63: 2549–2553
Schanberg SM, Schildkraut JJ, Breese GR, Kopin IJ (1968) Metabolism of normetanephrine-H-3 in rat brain. Identification of conjugated 3-methoxy-43-hydroxyphenylglycol as the major metabolite. Biochem Pharmacol 17: 247–254
Schweiger U, Warnhoff M, Pirke KM (1985) Brain tyrosine availability and the depression of central nervous norepinephrine turnover in acute and chronic starvation in adult male rats. Brain Res 335: 207–212
Schweiger U, Warnhoff M, Pirke KM (1985) Norepinephrine turnover in the hypothalamus of adult male rats, alteration of circadian patterns by semistarvation. J Neurochem 45: 706–709
Warnhoff M (1984) Simultaneous determination of norepinephrine, dopamine, 5-hydroxytryptamine, and their main metabolites in rat brain using high-performance liquid chromatography with electrochemical detection. Enzymatic hydrolysis of metabolites prior to chromatography. J Chromatogr 307: 217–281
Wurtman RJ, Hefti F, Melamed E (1981) Precursor control of neurotransmitter synthesis. Pharmacol Rev 32: 315–335
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Broocks, A., Liu, J. & Pirke, K.M. Semistarvation-induced hyperactivity compensates for decreased norepinephrine and dopamine turnover in the mediobasal hypothalamus of the rat. J. Neural Transmission 79, 113–124 (1990). https://doi.org/10.1007/BF01251006
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01251006