Summary
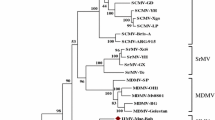
The N-terminal region of the coat proteins of five strains (Isis, Brisbane, Sabi, Bundaberg, and BC) of sugarcane mosaic virus (SCMV) isolated from four different plant species (sugarcane, sabi grass, wild sorghum, and blue couch grass) have been compared with the previously published data for SCMV-SC and SCMV-MDB, isolated from sugarcane and maize, respectively. The region, beginning at residue 11 and ending 16 residues beyond the second trypsin cleavage site of the coat protein, varied in size from 68 amino acid residues (Bundaberg) to 115 residues (BC) and contained repeat sequence motifs. Comparisons of the sequence identity and the nature of the repeats in the seven sequences showed that there were five different sequence patterns. These could be grouped further into three subsets which appeared to correlate with the host range of the strains. SCMV-Brisbane, SC, and Isis, isolated from sugarcane, showed almost identical sequence patterns and formed one subset. The other four strains had different sequence patterns and could be grouped further into a Sabi and Bundaberg subset (isolated from sabi grass), and a BC and MDB subset.
Similar content being viewed by others
References
Abbott EV, Tippett RL (1966) Strains of sugarcane mosaic virus. US Dept Agric Res Serv Tech Bull 1340, 25 pp
Barnett OW (1991) Potyviridae, a proposed family of plant viruses. Arch Virol 118: 139–141
Berger PH, Luciano CS, Thornbury DW, Hill JH, Zeyen RJ (1989) Properties and in vitro translation of maize dwarf mosaic virus RNA. J Gen Virol 70: 1845–1851
Frenkel MJ, Ward CW, Shukla DD (1989) The use of 3′ non-coding nucleotide sequence in the taxonomy of potyviruses: application to watermelon mosaic virus 2 and soybean mosaic virus. J Gen Virol 70: 2775–2783
Frenkel MJ, Jilka JM, McKern NM, Strike PM, Clark JM, Shukla DD, Ward CW (1991) Unexpected sequence diversity in the amino-terminal ends of the coat proteins of strains of sugarcane mosaic virus. J Gen Virol 72: 237–242
Frenkel MJ, Jilka JM, Shukla DD, Ward CW (1992) Differentiation of potyviruses and their strains by hybridization with the 3′ non-coding region of the viral genome. J Virol Methods 36: 51–62
Jensen SG, Staudinger JL (1989) Serological grouping of the cytoplasmic inclusions of six strains of sugarcane mosaic virus. Phytopathology 79: 1215
Jilka JM (1990) Cloning and characterization of the 3′-terminal regions of RNA from select strains of maize dwarf mosaic virus and sugarcane mosaic virus. PhD Thesis, University of Illinois, Urbana, Illinois
Lesemann DE, Shukla DD, Tosic M, Huth W (1992) Differentiation of the four viruses of the sugarcane mosaic virus complex based on cytopathology. In: Barnett OW (ed) Potyvirus taxonomy. Springer, Wien New York (Arch Virol [Suppl] 5), pp 353–361
Louie R, Knoke JK (1975) Strains of maize dwarf mosaic virus. Plant Dis Rep 59: 518–522
Matthews REF (1991) Plant virology, 3rd edn. Academic Press, New York
McKern NM, Wittaker LA, Strike PM, Ford RE, Jensen JG, Shukla DD (1990) Coat protein properties indicate that maize dwarf mosaic virus-KS 1 is a strain of Johnsongrass mosaic virus. Phytopathology 80: 907–912
McKern NM, Shukla DD, Toler RW, Jensen SG, Tosic M, Ford RE, Leon O, Ward CW (1991) Confirmation that the sugarcane mosaic virus subgroup consists of four distinct potyviruses by using peptide profiles of coat proteins. Phytopathology 81: 1025–1029
Pirone TP (1972) Sugarcane mosaic virus. CMI/AAB Description of Plant Viruses, no. 88
Shukla DD, Strike PM, Tracy SL, Gough KH, Ward CW (1988) The N- and C-termini of the coat proteins of potyviruses are surface-located and the N-terminus contains the major virus-specific epitopes. J Gen Virol 69: 1497–1508
Shukla DD, Tosic M, Jilka JM, Ford RE, Toler RW, Langham MAC (1989) Taxonomy of potyviruses infecting maize, sorghum, and sugarcane in Australia and the United States as determined by reactivities of polyclonal antibodies directed towards virusspecific N-termini of coat proteins. Phytopathology 79: 223–229
Shukla DD, Frenkel MJ, McKern NM, Ward CW, Jilka JM, Tosic M, Ford RE (1992) Present status of the sugarcane mosaic subgroup of potyviruses. In: Barnett OW (ed) Potyvirus taxonomy. Springer, Wien New York (Arch Virol [Suppl] 5), pp 363–373
Srisink S (1989) Studies on biological properties of sugarcane mosaic virus in Queensland. MSc Thesis, University of Queensland, St. Lucia, Queensland
Teakle DS, Grylls NE (1973) Four strains of sugarcane mosaic virus infecting cereals and other grasses in Australia. Aust J Agric Res 24: 465–477
Teakle DS, Shukla DD, Ford RE (1989) Sugarcane mosaic virus. CMI/AAB Descriptions of Plant Viruses, no. 342 (no. 88 revised)
Tosic M, Ford RE, Shukla DD, Jilka JM (1990) Differentiation of sugarcane, maize dwarf, Johnsongrass, and sorghum mosaic viruses based on reactions of oat and some sorghum cultivars. Plant Dis 74: 549–552
Ward CW, Shukla DD (1991) Taxonomy of potyviruses: current problems and some solutions. Intervirology 32: 269–296
Ward CW, Shukla DD (1993) Structure and variation of potyviruses. In: Rishi N, Singh BP (eds) Proc Int Conf Virol Trop Lucknow, India, Dec. 2–6, 1991 (in press)
Ward CW, McKern NM, Frenkel MJ, Shukla DD (1992) Sequence data as the major criterion for potyvirus classification. In: Barnett OW (ed) Potyvirus taxonomy. Springer, Wien New York (Arch Virol [Suppl] 5), pp 283–297
Ward CW, Weiller G, Shukla DD, Gibbs AJ (1993) Molecular evolution of potyviruses, the largest plant virus family. In: Gibbs AJ, Calisher CH, Garcia-Arenal F (eds) Molecular basis of virus evolution. Cambridge University Press, Cambridge (in press)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Xiao, X.W., Frenkel, M.J., Teakle, D.S. et al. Sequence diversity in the surface-exposed amino-terminal region of the coat proteins of seven strains of sugarcane mosaic virus correlates with their host range. Archives of Virology 132, 399–408 (1993). https://doi.org/10.1007/BF01309548
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01309548