Summary
Tomato plants were grown in nutrient culture either continuously or discontinuously treated with FeSO4 or with Fe-EDTA. FeSO4 rapidly gave a precipitate of Fe2O3.nH2O but remained, under the conditions of the experiments, available to the plants. Fe absorbed from Fe-EDTA in the nutrient medium was less effective in inducing tomato plant growth than iron supplied as ferrous sulphate. This cannot be explained in terms of luxury consumption or by phosphate-induced internal iron immobilization.
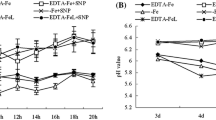
Plants grown continuously with Fe-EDTA as an iron source showed consistently higher peroxidase and catalase activities. Chlorotic plants treated for a few days with FeSO4 exhibited mostly equal or higher enzyme activities than those treated with Fe-EDTA. The ratio of green and yellow chloroplast pigments was constant under all the tested levels of iron nutrition.
Although there were significant differences in iron utilization, enzyme activities, and some symptoms of slightly modified growth, the main conclusion of this investigation is that, under these strictly comparable conditions, plant metabolism has not been modified considerably by the use of the synthetic iron chelates.
Similar content being viewed by others
References
Bailey, L. F. and McHargue, J. S., Effect of boron, copper, manganese and zinc on the enzyme activity of tomato and alfalfa grown in the greenhouse. Plant Physiol.19, 105–116 (1944).
Benedict, H. M., Simons, J. N., and Swidler, R., The absorption of iron and chelating agents by soybeans in split root experiments. Plant Physiol.37, proc. XLVII (1962).
Bennet, J. B., Iron in leaves, Soil Sci.60, 91–105 (1945).
Biddulph, O. and Woodbridge, C. G., The uptake of phosphorus by bean plants with particular reference to the effects of iron. Plant Physiol.27, 431–444 (1952).
Bolle-Jones, E. W. and Notton, B. A., The relative proportions of the chloroplast pigments as influenced by different levels of iron and potassium supply. Plant and Soil5, 87–100 (1953).
Branton, D. and Jacobson, L., Iron transportin pea plants. Plant Physiol.37, 539–545 (1962).
Branton, D. and Jacobson, L., Iron localization in pea plants. Plant Physiol.37, 546–551 (1962).
Brown, J. C., Iron chlorosis in plants. Advances Agron.13, 329–369 (1961).
Brown, J. C. and Tiffin, L. O., Properties of chelates and their use in crop production. J. Agr. Food Chem.10, 192–195 (1962).
Brown, J. C., Holmes, R. S., and Tiffin, L. O., Iron chlorosis in soybeans as related to the genotype of rootstalk: 3. Chlorosis susceptibility and reductive capacity at the root. Soil Sci.91, 127–132 (1961).
Brown, J. C., Tiffin, L. O., and Holmes, R. S., Competition between chelating agents and roots as factors affecting absorbtion of iron and other ions by plant species. Plant Physiol.35, 878–886 (1960).
Brown, J. C., Tiffin, L. O., Specht, A. W., and Resnicky, J. W., Iron absorption by roots as affected by plant species and concentration of chelating agent. Agron. J.53, 81–85 (1961).
Brown, J. C., Tiffin, L. O., Specht, A. W., and Resnicky, J. W., Stability and concentration of metal chelates, factors in iron chlorosis of plants. Agron. J.53, 85–90 (1961).
Burström, H. and Tullin, V., Observations on chelates and root growth. Physiol. Plantarum10, 406–417 (1957).
Burström, H., Growth action of EDTA in light and darkness. Physiol. Plantarum14, 354–377 (1961).
Chabareck, S. and Martell, A. E., in: Organic Sequestering Agents, New York, Wiley, p. 416–504 (1959).
Comar, C. L. and Zscheile, F. P., Analysis of plant extracts for chlorophylls a and b by a photoelectric spectrophotometric method. Plant Physiol.17, 198–209 (1942).
Davidson, D., The effect of chelating agents on cell division. Exp. Cell Research14, 329–332 (1958).
DeKock, P. C., The iron nutrition of plants at high pH. Soil Sci.79, 167–175 (1955).
DeKock, P. C., Commissiong, K., Farmer, V. C., and Inkson, R. H. E., Interrelationships of catalase, peroxidase, hematin and chlorophyll. Plant. Physiol.35, 599–604 (1960).
DeKock, P. C., Hall, A., and McDonald, M., A relation between the ratios of phosphorus to iron and potassium to calcium in mustard leaves. Plant and Soil12, 128–142 (1960).
DeKock, P. C. and Morrison, R. I., The metabolism of chlorotic leaves, I: Amino Acids. Biochem. J.70, 266–272 (1958).
Foreman, H., Use of chelating agents in treatment of metal poisoning (with special emphasis on lead). Federation Proc.20 (3), supplement no. 10, Part II, p. 191–196 (1961).
Hagen, C. E. and Jones, V. V., Factors in the determination of the intracellular localization of enzymes. Botan. Gaz.114, 130–134 (1952).
Hale, V. Q. and Wallace, A., Uptake by plants of59Fe vs.14C-chelating agents at different temperatures. pp. 53–57in: A Decade of Synthetic Chelating Agents in Inorganic Plant Nutrition, (1962) (Reference 50).
Haskins, F. A., Changes in the activities of several enzymes during germination and seedling development in corn (Zea Mays L.) Plant Physiol.30, 74–78 (1955).
Heath, O. V. S. and Clark, J. E., Chelation in auxin action; I: A study of the interactions of 3-indolylacetic acid and synthetic chelating agents as affecting the growth of wheat roots and coleoptile sections. J. Exp. Botany11, 167–187 (1960).
Hill-Cottingham, D. G., A spectrophotometric method of analysis of chelate solutions and its application to the study of iron chelates in soils and plants. Soil Sci.84, 43–50 (1957).
Hill-Cottingham, D. G. and Lloyd-Jones, C. P., Absortion and breakdown of iron-ethylenediaminetetraacetic acid by tomato plants. Nature189, 312 (1961).
Iljin, W. S., Metabolism of plants affected with lime-induced chlorosis: I Nitrogen metabolism. Plant and Soil3, 239–256 (1951).
Irving, H. and Williams, R. J. P., Order of stability of metal-complexes. Nature162, 746–747 (1948).
Jacobson, L., Maintenance of iron supply in nutrient solutions by a single addition of ferric potassium ethylenediaminetetraacetate. Plant Physiol.26, 411–413 (1951).
Jacobson, L. and Oertli, J. J., The relation between iron and chlorophyll contents in chlorotic sunflower leaves. Plant Physiol.31, 199–204 (1956).
Nason, A., Oldewurtel, H. A., and Propst, L. M., Role of micro-nutrient elements in the metabolism of higher plants: 1 Changes in the oxidative enzyme constituents of tomato leaves deficient in micro nutrient elements. Arch. Biochem. Biophys.38, 1–13 (1952).
Norton, G., The metabolism of plants affected by iron deficiency. University of Nottingham, Report of the school of agriculture 1961 (1962).
Olsen, C., Iron absorbtion in different plant species as a function of the pH value of the solution. Compt. Rend. Trav. Lab. Carlsberg31, 1–19 (1958).
Oosting, M., Enige beschouwingen in verband met de bepaling van sporenelementen in organisch materiaal. Analytische Instituut T.N.O., Rijswijk, The Netherlands, Mededeling58-1, 39 (1958).
Oser, B. L., Observations on the chronic ingestion of a chelating agent by rats and dogs. Federation Proc.20 (3) supplement no. 10, part II, 158 (1961).
Perur, N. G., Studies in iron chlorosis of leaves. Dissertation Utah University, Logan, Utah (1960).
Rameau, J. Th. L. B., and Have, J. ten, De bepaling van het gehalte aan fosforzuur in plantaardig materiaal en in de grond volgens de molybdeenblauwmethode met metol als reductiemiddel. Chem. Weekblad47, 1005–1013 (1951).
Schwarze, P., Beziehungen zwischen Peroxydase Reaktion, Eiweiszspiegel und Chlorophyllbildung. Planta44, 491–502 (1954).
Simons, J. N., Swidler, R., and Benedict, H. M., Absorbtion of chelated iron by soybean roots in nutrient solutions. Plant Physiol.37, 460–466 (1962).
Teng, D. and Miller, G. W., Biochemical differences associated with iron chlorosis in Glycine max. (Merr.) Plant Physiol.37 Proceed. XLVII (1962).
Thimann, K. V. and Takahashi, N., Interrelationships between metallic ions and auxin action, and the growth promoting action of chelating agents, pp. 363–380in: Plant Growth Regulation. Iowa State College Press. Ames (1960).
Tiffin, L. O., Brown, J. C., and Krauss, R. W., Differential absorbtion of metal chelate components by plant roots. Plant Physiol.35, 362–367 (1960).
Tiffin, L. O. and Brown, J. C., Selective absorbtion of iron from iron chelates by soybean plants. Plant Physiol.36, 710–714 (1961).
Trelease, S. F. and Trelease, H. M., Physiologically balanced culture solutions with stable hydrogen ion concentration. Science78, 438 (1933).
Vickery, H. B., Chemical investigations of the tobacco plant: XI Composition of the green leaf in relation to position on the stalk. Conn. Agr. Exp. Sta. New Haven Bull. 640 (1961).
Wallace, A., Use of synthetic chelatings agents in plant nutrition and some of their effects on carboxylating enzymes in plants. Ann. N.Y. Acad. Sci.88, 361–377 (1960).
Wallace, A., A Decade of Synthetic Chelating Agents in Inorganic Plant Nutrition, Wallace, A. 2278 Parnell Avenue, Los Angeles 64 California (1962).
Wallace, A. and Lunt, O. R., Iron chlorosis in horticultural plants, A review. Proc. Am. Soc. Hort. Sci.75, 819–841 (1960).
Wallace, A. and Hale, V. Q., Do chelating agents penetrate plant cells? pp. 57–62in: A decade of synthetic chelating agents in inorganic plant nutrition, (1962) (reference 51).
Weinstein, L. H., Meiss, A. M., Uhler, R. L., and Purvis, E. R., Growth-promoting effects of ethylenediaminetetraacetic acid. Nature178, 1188 (1956).
Weinstein, L. H. and Robbins, W. R., The effect of different iron and manganese nutrient levels on the catalase and cytochrome oxidase activities of green and albino sunflower leaf tissues. Plant Physiol.30, 27–32 (1955).
Westerfield, W. W., Effect of metal-binding agents on metallo-proteins. Federation Proc.20 (3), suppl. no. 10, 158–176 (1961).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Van Driel, W. The effect of iron ethylenediaminetetraacetic acid on the growth and metabolism of tomato plants in water culture. Plant Soil 20, 85–104 (1964). https://doi.org/10.1007/BF01378101
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01378101