Summary
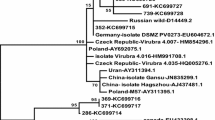
Detailed comparisons were made of the sequences of the coat protein (CP) cistrons and 3′-nontranslated regions (3′-NTR) of 21 (geographically) distinct isolates of potato virus Y (PVY) and a virus isolate initially described as pepper mottle virus (PepMoV). Multiple sequence alignments and phylogenetic relationships based on these alignments resulted into a subgrouping of virus isolates which largely corresponded with the historical strain differentiation based on biological criteria as host range, symptomatology and serology. Virus isolates belonging to the same subgroup shared a number of characteristic CP amino acid and 3′-NTR nucleotide residues indicating that, by using sequences from the 3′-terminal region of the potyvirus genome, a distinction could be made between different isolates of one virus species as well as between different virus species. RNA secondary structure analysis of the 3′-NTR of twelve PVY isolates revealed four major stem-loop structures of which, surprisingly, the loop sequences gave a similar clustering of isolates as resulting from the overall comparisons of CP and 3′-NTR sequences. This implies a biological significance of these structural elements.
Similar content being viewed by others
References
Abrahams JP, van den Berg M, Batenburg E, Pleij C (1990) Prediction of RNA secondary structure, including pseudoknotting, by computer simulation. Nucleic Acids Res 18: 3035–3044
Barnett OW (1991) Potyviridae, a proposed family of plant viruses. Arch Virol 18: 139–141
Barnett OW (1992) A summary of potyvirus taxonomy and definitions. In: Barnett OW (ed) Potyvirus taxonomy. Springer, Wien New York, pp 435–444 (Arch Virol [Suppl] 5)
Bowman-Vance V, Jordan R, Edwardson JR, Christie R, Purcifull DE, Turpen T, Falk B (1992 a) Evidence that pepper mottle virus and potato virus Y are distinct viruses: analyses of the coat protein and 3′ untranslated sequence of a California isolate of pepper mottle virus. In: Barnett OW (ed) Potyvirus taxonomy. Springer, Wien New York, pp 337–345 (Arch Virol [Suppl] 5)
Bowman-Vance V, Moore D, Turpen TH, Bracker A, Hollowel VC (1992) The complete nucleotide sequence of pepper mottle virus genomic RNA: comparison of the encoded polyprotein with those of other sequenced potyviruses. Virology 191: 19–30
Bravo-Almonacid F, Mentaberry AN (1989) Nucleotide cDNA sequence coding for the PVYo coat protein. Nucleic Acids Res 17: 4401
Carey J, Cameron V, de Haseth PL, Uhlenbeck OC (1983) Sequence-specific interactions of R 17 coat protein with its ribonucleic acid binding site. Biochemistry 22: 2601–2610
Cheong C, Varani G, Tinoco Jr I (1990) Solution structure of an unusually stable RNA hairpin, 5′GGAC(UUCG)GUCC. Nature 346: 680–682
Collmer CW, Stenzler L, Chen X, Fay N, Hacker D, Howell SH (1992) Single amino acid change in the helicase domain of the putative RNA replicase of turnip crinkle virus alters symptom intensification by virulent satellites. Proc Natl Acad Sci USA 89: 309–331
Culver JN, Dawson WO (1989) Point mutations in the coat protein gene of tobacco mosaic virus induce hypersensitivity in Nicotiana sylvestris. Mol Plant Microbe Interact 2: 209–213
Dalmay T, Balázs E (1990) Nucleotide sequence of an altered virulence potato virus Y coat protein gene (PVY-N strain). Nucleic Acids Res 18: 6721
De Bokx JA, Huttinga H (1981) Potato virus Y. CMI/AAB Descriptions of Plant Viruses, no. 242
Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12: 387–397
Dougherty WG, Allison RF, Parks TD, Johnston RE, Fields MJ, Armstrong FB (1985) Nucleotide sequence at the 3′ terminus of pepper mottle virus genomic RNA: evidence for an alternative mode of potyvirus capsid protein gene organization. Virology 146: 282–291
Eggen R, Verver J, Wellink J, Pleij K, Van Kammen A, Goldbach R (1989) Analysis of sequences involved in cowpea mosaic virus RNA replication using site-specific mutants. Virology 173: 456–464
Felstenstein J (1991)Phylip (phylogeny inference package) manual version 3.4. University of Washington, Seattle, U.S.A.
Feng S, Holland EC (1988) HIV-1tat trans-activation requires the loop sequence withintar. Nature 334: 165–167
Frenkel MJ, Ward CW, Shukla DD (1989) The use of 3′ non-coding nucleotide sequences in the taxonomy of potyviruses: application to watermelon mosaic virus 2 and soybean mosaic virus-N. J Gen Virol 70: 2775–2783
Grumet R, Fang G (1990) cDNA cloning and sequence analysis of the 3′-terminal region of zucchini yellow mosaic virus RNA. J Gen Virol 71: 1619–1622
Hataya T, Sano T, Ohshima K, Shikata E (1990) Polymerase chain reaction mediated cloning and expression of the coat protein gene of potato virus Y inEscherichia coli. Virus Genes 4: 339–350
Hay JM, Fellowes AP, Timmerman GM (1989) Nucleotide sequence of the coat protein gene of a necrotic strain of potato virus Y from New Zealand. Arch Virol 107: 111–122
Hiebert E, Purcifull DE (1992) A comparison of pepper mottle virus and potato virus Y and evidence for their distinction. In: Barnett OW (ed) Potyvirus taxonomy. Springer, Wien New York, pp 321–326 (Arch Virol [Suppl] 5)
Hikada M, Yoshida Y, Masaki H, Namba S, Yamashita S, Tsuchizaki T, Uozumi T (1992) Cloning and sequencing of the 3′ half of a potato virus Y (O-strain) genome encoding the 5K protein, protease, polymerase, and coat protein. Nucleic Acids Res 20: 3515
Holt CA, Hodgson RAJ, Coker FA, Beachy RN, Nelson RS (1990) Characterization of the masked strain of tobacco mosaic virus: identification of the region responsible for symptom attenuation by analysis of an infectious cDNA clone. Mol Plant Microbe Interact 3: 417–423
Ishikawa M, Meshi T, Watanabe Y, Okada Y (1988) Replication of ehimeric tobacco mosaic viruses which carry heterologous combinations of replicase genes and 3′ noncoding regions. Virology 164: 290–293
Jagadish MN, Ward CW, Gough KH, Tulloch PH, Whittaker LA, Shukla DD (1991) Expression of potyvirus coat protein inEscherichia coli and yeast and its assembly in virus-like particles. J Gen Virol 72: 1543–1550
Kamer G, Argos P (1984) Primary structural comparisons of RNA-dependent polymerases from plant, animal, and bacterial viruses. Nucleic Acids Res 9: 7269–7282
Lawson G, Kaniewski W, Haley L, Rozman R, Newell C, Sanders P, Tumer NE (1990) Engineering resistance to mixed virus infection in a commercial potato cultivar: resistance to potato virus X and potato virus Y in transgenic plants. Bio/Technology 8: 127–134
Meshi T, Motoyoshi F, Maeda T, Yoshiwoka S, Watanabe H, Okada Y (1989) Mutations in the tobacco mosaic virus 30-kD protein gene overcome Tm-2 resistance in tomato. Plant Cell 1: 515–522
Neeleman L, Van der Kuyl AC, Bol JF (1991) Role of alfalfa mosaic virus coat protein gene in symptom expression. Virology 181: 687–693
Oshima K, Hataya T, Sano T, Inoue AK, Shikata E (1991) Comparison of biological properties, serological characteristics and amino acid sequences of coat protein between potato virus Y ordinary strain and necrotic strain. Ann Phytopathol Soc Japan 57: 615–622
Petty ITD, Jackson AO (1990) Mutational analysis of barley stripe mosaic virus RNA β. Virology 179: 712–718
Puurand Ü, Saarma M (1990) Cloning and sequencing of the 3′-terminal region of potato virus Y-N (Russian isolate) RNA genome. Nucleic Acids Res 18: 6694
Rodríguez-Cerezo E, Gamble-Klein P, Shaw JG (1991) A determinant of disease symptom severity is located in the 3′-terminal noncoding region of the RNA of a plant virus. Proc Natl Acad Sci USA 88: 9863–9867
Robaglia C, Durand-Tardif M, Tronchet M, Bozadin G, Astier-Manifacier S, Casse-Delbart F (1989) Nucleotide sequence of potato virus Y (N strain) genomic RNA. J Gen Virol 70: 935–947
Rosner A, Raccah B (1988) Nucleotide sequence of the capsid protein gene of potato virus Y (PVY). Virus Genes 1: 255–260
Rybicki EP, Shukla DD (1992) Coat protein phylogeny and systematics of potyviruses. In: Barnett OW (ed) Potyvirus taxonomy. Springer, Wien New York, pp 139–170 (Arch Virol [Suppl] 5)
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406–425
Shukla DD, Ward CW (1988) Amino acid sequence homology of coat proteins as a basis for identification and classification of the potyvirus group. J Gen Virol 69: 7203–7210
Shukla DD, Thomas JE, McKern NM, Tracy SL, Ward CW (1988) Coat protein of potyviruses. 4. Comparison of biological properties, serological relationships, and coat protein amino acid sequences of four strains of potato virus Y. Arch Virol 102: 207–219
Shukla DD, Ward CW (1989 a) Identification and classification of potyviruses on the basis of coat protein sequence data and serology. Arch Virol 106: 171–200
Shukla DD, Ward CW (1989 b) Structure of potyvirus coat proteins and its application in the taxonomy of the potyvirus group. Adv Virus Res 36: 273–314
Shukla DD, Frenkel MJ, Ward CW (1991) Structure and function of the potyvirus genome with special reference to the coat protein coding region. Can J Plant Pathol 13: 178–191
Takamatsu N, Watanabe Y, Meshi T, Okada Y (1990) Mutational analysis of the pseudoknot region in the 3′ noncoding region of tobacco mosaic virus RNA. J Virol 64: 3686–3693
Tuerk C, Gauss P, Thermes C, Groebe DR, Gayle M, Guild N, Stormo G, D'Aubenton-Carafa Y, Uhlenbeck OC, Tinoco I Jr, Brody EN, Gold L (1988) CUUCGG hairpins: extraordinarily stable RNA secondary structures associated with various biochemical processes. Proc Natl Acad Sci USA 85: 1364–1368
Van der Vlugt RAA, Allefs S, de Haan PT, Goldbach RW (1989) Nucleotide sequence of the 3′-terminal region of the potato virus Y-N RNA. J Gen Virol 70: 229–233
Van der Vlugt RAA (1992) Is pepper mottle virus a strain of potato virus Y? In: Barnett OW (ed) Potyvirus taxonomy. Springer, Wien New York, pp 327–335 (Arch Virol [Suppl] 5)
Varani G, Cheong C, Tinoco I Jr (1991) Structure of an unusually stable hairpin loop. Biochemistry 30: 3280–3289
Ward CW, Shukla DD (1991) Taxonomy of potyviruses: current problems and some solutions. Intervirology 32: 269–296
Ward CW, McKern NM, Frenkel MJ, Shukla DD (1992) Sequence data as the major criterion for potyvirus classification. In: Barnet OW (ed) Potyvirus taxonomy. Springer, Wien New York, pp 283–297 (Arch Virol [Suppl] 5)
Wefels E, Sommer H, Salamini F, Rohde W (1989) Cloning of the potato virus Y genes encoding the capsid protein and the nuclear inclusion protein NIb. Arch Virol 107: 123–134
Westhof E, Dumas P, Moras D (1985) Crystallographic refinement of Yeast aspartic acid transfer RNA. J Mol Biol 184: 119–145
Witherell GW, Uhlenbeck OC (1989) Specific RNA binding of Qβ coat protein. Biochemistry 28: 71–76
Woese CR, Winker S, Gutell RR (1990) Architecture of ribosomal RNA: constraints on the sequence of “tetra-loops”. Proc Natl Acad Sci USA 87: 8467–8471
Zhou X-R, Fang R-X, Chen Z-X, Mang K-Q (1990) cDNA sequence of the 3′-coding region of PVY genome (the Chinese isolate). Nucleic Acids Res 18: 5554
Zitter TA (1972) Naturally occurring pepper virus strains in Florida. Plant Dis Rep 56: 586–590
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van der Vlugt, R.A.A., Leunissen, J. & Goldbach, R. Taxonomic relationships between distinct potato virus Y isolates based on detailed comparisons of the viral coat proteins and 3′-nontranslated regions. Archives of Virology 131, 361–375 (1993). https://doi.org/10.1007/BF01378638
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01378638