Summary
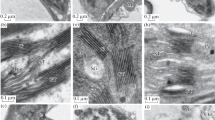
We studied cell ultrastructure and carbohydrate levels in the leaf tissue ofArabidopsis thaliana L. (Heyn) cv. Columbia during rapid cold acclimation. Freezing tolerance of the leaves from 26 day old plants was determined after 48 h and 10 days at 4°C. Acclimation treatment of 48 h decreased the lethal freezing temperature from −5.7°C to −9.4°C. Freezing tolerance was not altered further by acclimation at 4 °C for 10 days. Ultrastructural changes in the parenchyma cells were evident after 6 to 24 h of cold acclimation. The plasma membrane showed signs of extensive turnover. Evidence of membrane invaginations and sequestering of membrane material was observed. In addition, numerous microvesicles, paramural bodies, and fragments of endoplasmic reticulum were noticed in the vicinity of plasma membrane. Modifications in the structure of cell membranes were evident after 5 days of exposure to low temperature. Small, darkly stained globules were seen on the plasma membrane, tonoplast, chloroplast envelope membrane, mitochondrion outer membrane, dictyosome cisternae membrane, and microvesicle membrane. As far as we are aware, this type of membrane modification has not been described previously in plant cells exposed to low temperature. We propose to call these structures membraglobuli. Acclimation treatment also increased the concentrations of soluble sugars and starch. These observations suggest that cold acclimation inA. thaliana induces changes in both plasma membrane properties and carbohydrate composition.
Similar content being viewed by others
References
Guy CL (1990) Cold acclimation and freezing stress tolerance: role of protein metabolism. Annu Rev Plant Physiol Plant Mol Biol 41: 187–223
Gilmour SJ, Hajela RK, Thomashow MF (1988) Cold acclimation inArabidopsis thaliana. Plant Physiol 87: 745–750
Goldstein G, Nobel PS (1991) Changes in osmotic pressure and mucilage during low-temperature acclimation ofOpuntia flcusindica. Plant Physiol 97: 954–961
James G (1976) Mathematics dictionary, 4th edn. Van Nostrand Reinhold, New York
Koster KL, Lynch DV (1992) Solute accumulation and compartmentation during the cold acclimation of puma rye. Plant Physiol 98: 108–113
Kurkela S, Franck M, Heino P, Lang V, Palva ET (1988) Cold induced gene expression inArabidopsis thaliana L. Plant Cell Rep 7: 495–498
Levitt J (1980) Responses of plants to environmental stress, vol 1. Academic Press, New York
Lin C, Guo WW, Everson E, Thomashow MF (1990) Cold acclimation inArabidopsis and wheat. Plant Physiol 94: 1078–1083
Marchant R, Robards AW (1968) Membrane systems associated with the plasmalemma of plant cells. Ann Bot 32: 457–471
Niki T, Sakai A (1981) Ultrastructural changes related to frost hardiness in the cortical parenchyma cells from mulberry twigs. Plant Cell Physiol 22: 171–183
— — (1982) Effect of cycloheximide on the freezing tolerance and ultrastructure of cortical parenchyma cells from mulberry twigs. Can J Bot 61: 2205–2211
Perras M, Sarham F (1984) Energy state of spring and winter wheat during cold hardening. Soluble sugars and adenine nucleotides. Physiol Plant 60: 129–132
Pomeroy MK, Siminovitch D (1971) Seasonal cytological changes in secondary phloem parenchyma cells inRobinia pseudoacacia in relation to cold hardiness. Can J Bot 49: 787–795
Pollock CJ, Lloyd EJ (1987) The effect of low temperature upon starch, sucrose, and fructan synthesis in leaves. Ann Bot 60: 231–235
— —, Stoddart JL, Thomas H (1983) Growth, photosynthesis, and assimilate partitioning inLolium temulentum exposed to chilling temperatures. Physiol Plant 59: 257–262
Ristic Z, Cass DD (1991) Morphological characteristics of leaf epidermal cells in lines of maize that differ in endogenous levels of abscisic acid and drought resistance. Bot Gaz 152: 439–445
— — (1992) Chloroplast structure after water and high-temperature stress in two lines of maize that differ in endogenous levels of abscisic acid. Int J Plant Sci 153: 186–196
Robinson DG, Quader H (1981) Structure, synthesis, and orientation of microfibrils. IX. A freeze-fracture investigation of theOocystis plasma membrane after inhibitor treatments. Eur J Cell Biol 25: 278–288
Rose R, Rose CH, Omi SK, Forry KR, Durall DM, Bigg WL (1991) Starch determination by perchloric acid vs enzymes: evaluating the accuracy and precision of six colorimetric methods. J Agric Food Chem 39: 2–11
Sakai A, Larcher N (1987) Frost survival in plants. Responses and adaptations to freezing stress. Springer, Berlin Heidelberg New York Tokyo
Sauter JJ, Cleve BV (1991) Biochemical and ultrastructural results during starch-sugar-conversion in ray parenchyma cells ofPopulus during cold adaptation. J Plant Physiol 139: 19–26
—, Kloth S (1987) Changes in carbohydrates and ultrastructure in xylem ray cells ofPopulus in response to chilling. Protoplasma 137: 45–55
Shi J, Mueller WC, Beckman CH (1991 a) Ultrastructural responses of vessel contact cells in cotton plants resistant or susceptible to infection byFusarium oxysporum f. sp.vasinfectum. Physiol Mol Plant Pathol 38: 211–222
— — — (1991 b) Ultrastructure and histochemistry of lipoidal droplets in vessel contact cells and adjacent parenchyma cells in cotton plants infected byFusarium oxysporum f. sp.vasinfectum. Physiol Mol Plant Pathol 39: 201–211
Smith D (1981) Removing and analyzing total nonstructural carbohydrates from plant tissue. Wisc Agric Res Rep R 2107
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26: 31–43
Steer MW (1988) Plasma membrane turnover in plant cells. J Exp Bot 39: 987–996
Steponkus PL (1984) Role of the plasma membrane in freezing injury and cold acclimation. Annu Rev Plant Physiol 35: 543–584
—, Dowgert MF, Gordon-Kamm WJ (1983) Destabilization of the plasma membrane of isolated plant protoplasts during a freezethaw cycle: the influence of cold acclimation. Cryobiology 20: 448–465
Sukumaran NP, Weiser CJ (1972) An excised leaflet test for evaluating potato frost tolerance. HortScience 7: 461–468
Tolbert NE (1971) Microbodies — peroxisomes and glyoxysomes. Annu Rev Plant Physiol 22: 45–74
Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 6: 24
Uemura M, Yoshida S (1984) Involvement of plasma membrane alterations in cold acclimation of winter rye seedlings (Secale cereale L. cv. Puma). Plant Physiol 75: 818–826
Van Handel E (1968) Direct microdetermination of sucrose. Anal Biochem 22: 280–283
Venable JH, Coggeshall R (1965) A simplified lead citrate stain for use in electron microscopy. J Cell Biol 25: 407–408
Wisniewki M, Ashworth EN (1986) A comparison of seasonal ultrastructural changes in stem tissues of peach (Prunus persica) that exhibit contrasting mechanisms of cold hardiness. Bot Gaz 147: 407–417
Yoshida S (1984) Chemical and biophysical changes in the plasma membrane during cold acclimation of mulberry bark cells (Morus bombycus Koidz. cv. Goroji). Plant Physiol 76: 257–265
—, Uemura M (1984) Protein and lipid compositions of isolated plasma membranes from orchard grass (Dactylis glomerta L.) and changes during cold acclimation. Plant Physiol 75: 31–37
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ristic, Z., Ashworth, E.N. Changes in leaf ultrastructure and carbohydrates inArabidopsis thaliana L. (Heyn) cv. Columbia during rapid cold acclimation. Protoplasma 172, 111–123 (1993). https://doi.org/10.1007/BF01379368
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01379368