Abstract
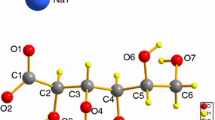
The crystal structure of one of the hydrated forms of nedocromil sodium, the heptahemihydrate, has been determined. Crystal data:a=26.687(5),b=29.479(8),c=6.394(3) Å, orthorhombic, space groupAba2,Z=8. This structure is compared with the previously determined structure of the trihydrate [Freer, A. A., Payling, D. W., and Suschitzky, J. L.,Acta Crystallogr. 1987,C43, 1900–1905]. The two structures differ in the molecular conformation of the nedocromil anion, the intensity of color, the coordination of the sodium ions, and the environment of the water molecules. In the heptahemihydrate two of the water molecules from dimers, whereas the remaining water molecules form a two-dimensional network consisting of a pentameric chain in one direction and an infinite chain in the other. From another viewpoint, 5 1/2 water molecules in the heptahemidydrate are present in the first coordination sphere of the sodium ions, whereas the remaining two water molecules are present as “lattice” water in the crystal.
Similar content being viewed by others
References
Haleblian, J.; Macrone, W.J. Pharm. Sci. 1969,58, 911–929.
Haleblian, J.J. Pharm. Sci. 1975,64, 1269–1288.
Zografi, G.; Kontny M.Pharm. Res. 1986,3, 187–194.
Khankari R. K.; Grant, D. J. W.Thermochim. Acta 1995,248, 61–69.
Kontny, M.Drug Dev. Ind. Pharm. 1988,14, 1991–2027.
Cairns, H.; Cox, D.; Gould, K. J.; Ingall, A. H.; Suschitzky, J.L.J. Med. Chem. 1985,28, 1832–1842.
Freer, A. A.; Payling, D. W.; Suschitzky, J. L.Acta Crystallogr. 1987,C 43, 1900–1905.
Khankari, R. K.; Chan, H.-K.; Grant, D. J. W..Pharm. Res. 1989,6, S-47.
Khankari, R. K.; Law, D.; Grant, D. J. W..Pharm. Res. 1990,7, S-108.
Khankari, R. K.; Law, D.; Grant, D. J. W.Int. J. Pharm 1992,82, 117–127.
Khankari, R. K.; Grant, D. J. W.Pharm. Res. 1993,19, S-153.
Khankari, R. K.; Grant, D. J. W.Pharm. Res. 1994,11, S-241.
Shefter, E.; Higuchi, T. Dissolution behavior of crystalline solvated and nonsolvated forms of some pharmaceuticals.J. Pharm. Sci. 1963,52, 781–791.
Sheldrick, G. M..SHELXS86: Program for the Solution of Crystal Structures; University of Göttingen, Germany, 1986.
TEXSAN-TEXRAY Structure Analysis Package, Molecular Structure Corporation. The Woodlands, TX, 1985.
International Tables for X-ray Crystallography, Vol. IV; Kynoch Press: Birmingham, England, 1974.
Johnson, C. K.ORTEP II. Report ORNL-5138; Oak Ridge National Laboratory, Oak Ridge, TN, 1976.
Spek, A. L.PLUTON Program for Molecular Graphics: Utrecht University, The Netherlands, 1992.
Rys, P.; Zollinger, H.Fundamentals of the Chemistry and Application of Dyes; Wiley-Interscience: London, 1972.
Griffiths, J.Colour and Constitution of Organic Molecules: Academic Press: London, 1976.
Hickey, A.; Martonen, T.Pharm. Res. 1993,10, 1–7.
Byrn, S. R.Solid State Chemistry of Drugs; Academic Press: New York, 1982.
Falk, M.; Knop, O.Water, a Comprehensive Treatise; Vol. 2, Plenum: New York, 1973.
Whyte, S.; Webster, B. B.Sc. Project Thesis, Chemistry Department, University of Glasgow, UK, 1993.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Khankari, R.K., Ojala, W.H., Gleason, W.B. et al. Crystal structure of nedocromil sodium heptahemihydrate and its comparison with that of nedocromil sodium trihydrate. J Chem Crystallogr 25, 863–870 (1995). https://doi.org/10.1007/BF01671084
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01671084