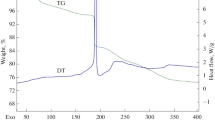
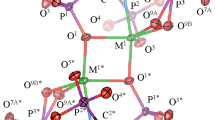
Abstract
The paper deals with the chemical and physical factors influencing the stoichiometry of thermal decomposition of solid coordination compounds. Nickel(II) coordination compounds were used as examples: the problem of the relationship between the structure of the initial compound (and of its intermediates) and the stoichiometry of thermal decomposition is discussed; experimental conditions are shown to affect this decomposition, and the conceptions of the apparent and real stoichiometries of thermal decomposition are discussed. The results obtained may have a more general meaning.
Résumé
L'article considère les facteurs chimiques et physiques qui influencent la st∄chiométrie des réactions de décomposition thermique des composés de coordination solides. Les composés de coordination du nickel(II) ont été choisis pour illustrer le problème des relations entre la structure du composé initial (et de ses intermédiaires) et la st∄chiométrie de la réaction de décomposition thermique. On montre que les conditions expérimentales influencent la décomposition et on discute le principe des réactions st∄chiométriques de décomposition thermique dites «apparentes» et «réelles». Les résultats obtenus sont probablement de portée plus générale.
Zusammenfassung
Der Beitrag befa\t sich mit den chemischen und physikalischen Faktoren, welche die Stöchiometrie der thermischen Zersetzung fester Koordinationsverbindungen beeinflussen. Koordinationsverbindungen von Nickel(II) wurden als Beispiel eingesetzt; das Problem des Zusammenhanges zwischen Struktur der Ausgangsverbindung (und seiner IntermediÄrprodukte) und der Stöchiometrie der thermischen Zersetzung wird erörtert; die die Zersetzung beeinflussenden Versuchsbedingungen werden gezeigt und das Konzept der sogenannten scheinbaren und tatsÄchlichen Stöchiometrie der thermischen Zersetzung wird beschrieben. Die erhaltenen Ergebnisse dürften von allgemeinerer Bedeutung sein.
РЕжУМЕ
В стАтьЕ РАссМАтРИВА Утсь хИМИЧЕскИЕ И ФИжИЧЕскИЕ ФАктОРы, ж АтРАгИВАУЩИЕ стЕхИОМЕтРИУ тЕРМИЧ ЕскОгО РАжлОжЕНИь тВ ЕРДых кООРДИНАцИОННых сОЕ ДИНЕНИИ. НА пРИ$ МЕРЕ кООРДИНАцИОННы х сОЕДИНЕНИИ НИкЕль(II) БылА ОБсУжДЕНА ВжАИМОсВь жь МЕжДУ стРУк$ тУРОИ НАЧАльНОгО сОЕ ДИНЕНИь (И ЕгО пРОМЕжУ тОЧНых пРОДУктОВ) И стЕхИОМЕ тРИЕИ тЕРМИ$ ЧЕскОгО РАжлОжЕНИь. п ОкАжАНы ЁкспЕРИМЕНт АльНыЕ УслОВИь, жАтРАгИВАУЩ ИЕ ЁтО РАжлО$ жЕНИЕ, И ОБсУжДЕНА кОН цЕпцИь тАк НАжыВАЕМО И кАжУЩЕИсь И ИстИННОИ стЕхИОМЕт РИИ тЕРМИ$ ЧЕскОгО РАжлОжЕНИь. п ОлУЧЕННыЕ РЕжУльтАт ы МОгУт ИМЕть БОлЕЕ ОБЩЕЕ жНА ЧЕНИЕ.
Similar content being viewed by others
References
W. W. Wendlandt andJ. P. Smith, The Thermal Properties of Transition Metal Ammine Complexes, Elsevier, Amsterdam, 1967.
G. Liptay, Thermochim. Acta, 15 (1976) 159.
E. Uhlig, Coord. Chem. Rew., 10 (1973) 227.
A. BlaŽek, Thermal Analysis, Van Nostrand Reinhold Co., London, 1973.
M. A. Poraj-Koshits andA. C. Ancyskhina, Kristallografija, 3 (1958) 686.
E. Jona, B. Vojtas, T. šramko andJ. GaŽo, Chem. Zvesti, 30 (1976) 100.
E. Jóna, B. Vojtas andT. šramko, Chem. Zvesti, 30 (1976) 107.
R. C. Evans, An Introduction to Crystal Chemistry, p. 298, Cambridge University Press, London, 1966.
S. Sarig, Thermochim. Acta, 7 (1973) 297.
R. Rabbering, J. Wanrooy andA. Scuff, Thermochim. Acta, 12 (1975) 57.
H. Chihara andS. Seki, J. Chem. Soc. Japan, 26 (1953) 88.
S. Caillère andT. Pobequin, Bull. Soc. Chim. France, Miner. Crist., 85 (1962) 48.
G.Pannetier, J. M.Brégeault, C.Laconturier and G.Djegamariadasson, Bull. Soc. Chim. France (1964) 1141.
E. Jóna andT. šramko, J. Thermal. Anal., 12 (1977) 91.
T. šramko, G. Liptay andE. Jóna, J. Thermal. Anal., 12 (1977) 217.
F. Paulik andJ. Paulik, J. Thermal. Anal., 5 (1973) 253.
E. Jóna, T. šramko, J. Kohout, A. Sirota andJ. GaŽo, Chem. Zvesti, 25 (1971) 241.
E. Jóna, T. šramko andJ. Gazo, J. Thermal. Anal., 7 (1975) 551.
T. Sramko andE. Jóna, Collect. Czech. Chem. Commun., 37 (1972) 1645.
T.Sramko, E.ona and G.Liptay, Proceedings of the 5th Conference on Coordination Chemistry, Smolenice-Bratislava, p. 251.
E. Jóna, T. šramko andJ. GaŽo, J. Thermal Anal., 4 (1972) 61.
Atlas of thermoanalytical curves, ed. by G. Liptay, Akadémiai Kiadó (Budapest), 2 (1973) 83.
G. A. Evlanov, Izv. Vysshykh Uchebn. Zavedenii Khim. i Khim. Tekhnol., 6 (1963) 3.
J. G. Murgulescu, E. Segal andD. Fatu, J. Inorg. Nucl. Chem., 27 (1965) 2677.
E. Jóna andT. šramko, Chem. Zvesti, 20 (1966) 569.
E. Jóna andT. šramko, Chem. Zvesti, 21 (1967) 517.
J. Kohout, M. KohÚtová andE. Jóna, Z. Naturforsch., 25 (1970) 1054.
E. ďurčanská andJ. Garaj, Inorg. Chim. Acta, 29 (1978) 149.
E. Jóna, T. šramko andJ. GaŽo, Chem. Zvesti, 27 (1973) 145.
E. Jóna, T. šramko andJ. GaŽo, Chem. Zvesti, 27 (1973) 588.
E. Jóna, M. Jamnický, T. šramko andJ. GaŽo, Collect. Czech. Chem. Commun., 37 (1972) 3679.
B. Vojtas, E. Jóna andT. šramko, Z. Chem., 14 (1974) 445.
E. ďurčanská, M. Koman andE. Jona, Z. Chem., 16 (1976) 453.
E. Jóna, P. Ambrovič, T. šramko andJ. GaŽo, J. Thermal Anal., 4 (1972) 153.
E. Jóna, M. Jamnický, and T. Sramko, Z. Anorg. Allgem. Chem, 447 (1978) 207.
G. Liptay, Kémiai Közlemények, 32 (1969) 389.
D. H. Brown, R. N. Nuttall andD. W. A. Sharp, J. Inorg. Nucl. Chem., 26 (1964) 1151.
J. R. Allan, D. H. Brown, R. H. Nuttall andD. W. A. Sharp, J. Inorg. Nucl. Chem., 27(1965)1529.
Author information
Authors and Affiliations
Additional information
Reported at the 7th Seminar on Modern Methods in Inorganic Chemistry. Harmonia-Bratislava, 1977.
Rights and permissions
About this article
Cite this article
Jóna, E., šramko, T. & GaŽo, J. Thermal properties of solid nickel(II) coordination compounds. Journal of Thermal Analysis 16, 213–229 (1979). https://doi.org/10.1007/BF01909649
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01909649