Abstract
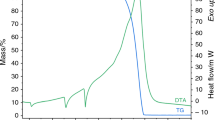
Nineteen salts of hexachloroplatinic acid with monovalent metals, aromatic and heterocyclic amines and phosphine were obtained and characterized by chemical analysis. The thermal decomposition of these complexes was studied by means of derivatograph and differential scanning calorimetry. The nature of the pyrolysis processes is discussed. From the TG curves, kinetic parameters were derived for different stages of the thermal decompositions.
Zusammenfassung
Es wurden neunzehn Salze von Hexachlorplatinsäure mit monovalenten Metallen, aromatischen und heterocyclischen Aminen und Phosphinen hergestellt und mittels chemischer Analyse beschrieben. Mittels Derivatographie und DSC wurden die thermischen Eigenschaften dieser Komplexe untersucht. Es wird die Natur des Pyrolyseprozesses diskutiert. Anhand der TG-Kurven wurden kinetische Parameter für die einzelnen Schritte der thermischen Zersetzung ermittelt.
Similar content being viewed by others
References
S. M. Jörgensen, J. Prakt. Chem., 30 (1884) 14; ibid. 31 (1885) 80.
P. Pfeiffer and G. Lando, Ber. deutsch. chem. Ges., 37 (1904) 4282.
Gmelin's Handbuch der anorganischen Chemie, Kobalt, Syst. No. 58, Teil B, Verlag Chemie, GMBH, Weinheim 1956, pp. 71, 72, 87, 112, 113, 139.
W. Peters, Z.anorg. allg. Chem., 89 (1914) 191.
H. D. K. Drew and H. J. Tress, J. Chem. Soc., (1933) 1341.
A. Ladenburg, Ber. deutsch. chem. Ges., 21 (1886) 286.
Gmelin's Handbuch der anorganischen Chemie, 8 Aufl. Platin, System No. 68, Teil C Lief. 2, Verlag Chemie, GMBH, Weinheim 1940, p. 226.
J. Meyer and H. Kienitz, Z. anorg. allg. Chem., 242 (1939) 296.
A. Partheil and A. van Haaren, Archiv Pharm., 238 (1900) 42.
W. C. J. Dyke, G. Davies and W. J. Jones, J. Chem. Soc., (1931) 187.
A. Werner and F. Fassbänder, Z.anorg allg. Chem., 15 (1897) 123.
F. Basolo, J. C. Bailar and B. R. Tarr, J. Amer. Chem. Soc., 72 (1950) 2433.
R. H. Atkinson, Trans. Faraday Soc., 26 (1930) 492.
F. Puche, Ann. Chim., 9 (1938) 234, 257.
M. Delépine, Ann. Chim., 7 (1917) 291.
A. Gutbier, P. Heinrich, L. V. Müller and J. Liebers, Z. anorg. allg. Chem., 81 (1913) 379.
J. Zsakó, J. Thermal Anal., 15 (1979) 369.
J. Zsakó, M. Várhelyi and Cs. Várhelyi, J. Thermal Anal., 17 (1979) 123.
P. D. Garn, J. Thermal Anal., 10 (1976) 99.
A. J. Lesnikovich and S. V. Levchik, J. Thermal Anal., 30 (1985) 677.
J. Zsakó, J. Sztatisz, A. Czégeni, G. Liptay and Cs. Várhelyi, J. Thermal Anal., 32 (1987) 463.
Cs. Várhelyi, J. Zsakó, G. Liptay and Z. Finta, J. Thermal Anal., 32 (1987) 785.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zsakó, J., Liptay, G., Várhelyi, C. et al. Kinetic analysis of thermogravimetric data. Journal of Thermal Analysis 37, 2681–2691 (1991). https://doi.org/10.1007/BF01912812
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01912812