Summary
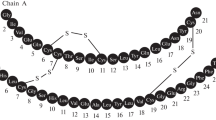
Eel-calcitonin and its [Asu1,7]-analog, deamino-dicarba-analog, were synthesized by the conventional solution method. The natural-type product showed 4300 MRC U/mg in the hypocalcemic potency which was comparable to that of the natural hormone isolated from eel. Hormonal activity of the Asu-analog was also as high as 3400 MRC U/mg.
Similar content being viewed by others
References
M. Otani, T. Noda, H. Yamauchi, S. Watanabe, T. Matsuda, H. Orimo andK. Narita, Proc. 5th Parathyroid Conference, Oxford (Eds.R. V. Talmage, M. Owen andJ. A. Parsons; Excerpta Medica 1974), p. 111. —M. Otani, H. Yamauchi, T. Meguro, S. Kitazawa, S. Watanabe andH. Orimo, J. Biochem., Tokyo79, 345 (1976).
T. Noda andK. Narita, J. Biochem. Tokyo79, 353 (1976).
H. D. Niall, H. T. Keutmann, D. H. Copp andJ. T. Potts, Jr., Proc. natn. Acad. Sci., USA64, 771 (1969).
R. K. O'Dor, C. O. Parkes andD. H. Copp, Can. J. Biochem.47, 823 (1969).
H. T. Keutmann, J. A. Parsons, J. T. Potts, Jr. andR. J. Schlueter, J. biol. Chem.245, 1491 (1970).
St.Guttmann, J. Pless, R. L. Huguenin Ed. Sandrin, H. Bossert andK. Zehnder, Helv. chim. Acta52, 1789 (1969).
K. Jôst andJ. Rudinger, Collect. Czech. chem. Commun.32, 1229 (1967).
S. Sakakibara andS. Hase, Bull. chem. Soc. Japan41, 2816 (1968).—A. Kobayashi, S. Hase, R. Kiyoi andS. Sakakibara, Bull. chem. soc. Japan42, 3491 (1969). —S. Hase, T. Morikawa andS. Sakakibara, Experientia25, 1239 (1969).
Amino acids except Gly denote thel-configuration. Abbreviations are in accordance with the recommendations of IUPAC-IUB Commission (J. biol. Chem.247, 977 (1972). Z(Cl), 2-Chlorobenzyloxycarbonyl; HOSu,N-Hydroxysuccinimide; Bzl(Cl2), 2,6-Dichlorobenzyl; MBzl,p-Methoxybenzyl; TFA, Trifluoroacetic acid; DMF,N,N-Dimethylformamide; A, C2H5−N=C=N−(CH2)3−N(CH3)2/1-Hydroxybenztriazole method; B, DCC/HOSu method. The Boc group in each fragment was removed by TFA.
S. Sakakibara andY. Shimonishi, Bull. chem. Soc. Japan38, 1412 (1965).—S. Sakakibara, Y. Shimonishi, Y. Kishida, M. Okada andH. Sugihara, Bull. chem. Soc. Japan40, 2164 (1967).
M. A. Kumar, E. Slack, A. Edwards, H. A. Soliman, A. Baghdiantz, G. V. Foster andI. MacIntyre, J. Endocr.33, 469 ( (1965).
W. Rittel, R. Maier, M. Brugger, B. Kamber, B. Riniker andP. Sieber, Experientia32, 246 (1976).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morikawa, T., Munekata, E., Sakakibara, S. et al. Synthesis of Eel-Calcitonin and [Asu1,7]-Eel-Calcitonin: Contribution of the disulfide bond to the hormonal activity. Experientia 32, 1104–1106 (1976). https://doi.org/10.1007/BF01927568
Issue Date:
DOI: https://doi.org/10.1007/BF01927568