Summary
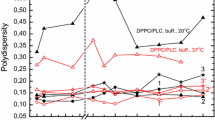
An electron spin probe study was made of the effect of a number of mitogenic agents on the ordering and state of aggregation of the plasma membrane lipids of lymphocytes. These agents, which included phytohemagglutinin, Concanavalin A, the calcium ionophore A23187 and periodate, caused a 20% decrease in lipid ordering in the region of the bilayer probed by 5-nitroxide stearic acid. The corresponding methyl ester probe showed marked probe-probe interaction under the same conditions indicating an aggregation of lipids in the area probed by this label. Studies with mixed lipid vesicles and gangliosidefree cells indicate that these areas are rich in glycolipids capable of hydrogen bonding to the ester probe. The decrease in ordering and the increase in aggregation of the membrane lipids were correlated with the patching and capping of the ligand-receptor complexes. Furthermore, the disappearance of fluorescent ligand from the surface of treated cells corresponded with the return of the spectral parameters of the probes to control cell values.
It was concluded that glycolipids might play an important role in ligand-induced cell surface changes either as bearers of receptor groups, as in the case of some gangliosides, or in association by hydrogen-bonding with receptor proteins.
Similar content being viewed by others
References
Allwood, G., Asherson, G., Davey, M.J., Goodford, P. 1971. The early uptake of radioactive calcium by human lymphocytes treated with phytohaemagglutinin.Immunology 21:509
Barnett, R.E., Scott, R.E., Furcht, L.T., Kersey, J.H. 1974. Evidence that mitogenic lectins induce changes in lymphocyte membrane fluidity.Nature (London) 249:465
Beppu, M., Terao, T., Osawa, T. 1976. Preparation of monovalent succinyl-concanavalin A and its mitogenic activity.J. Biochem. (Tokyo) 79:1113
Berg, K.J. van den, Betel, I. 1973. Increased transport of 2-aminoisobutyric acid in rat lymphocytes stimulated with concanavalin A.Exp. Cell Res. 76:63
Berg, K.J. van den, Betel, I. 1974. Regulation of amino acid uptake in lymphocytes stimulated by mitogens. In. Increase in AIB transport dependent on cell metabolism.Exp. Cell Res. 84:412
Böyum, A. 1968. Isolation of leucocytes from human blood.Scand. J. Clin. Lab. Invest. 21 (Suppl. 97):9
Craig, S.W., Cuatrecasas, P. 1975. Mobility of cholera toxin receptors on rat lymphocyte membranes.Proc. Nat. Acad. Sci. USA 72:3844
Critchley, D.R., McPherson, I. 1973. Cell density dependent glycolipids in NIL2 hamster cells, derived malignant and transformed cell lines.Biochim. Biophys. Acta 296:145
Curtain, C.C., Anderson, N. 1972. Parasite antigens and host antibodies inOstertagia circumcinta infection of the sheep.Int. J. Parasitol. 2:449
Devaux, P., McConnell, H.M. 1972. Lateral diffusion in spin-labeled phosphatidylcholine multilayers.J. Am. Chem. Soc. 94:4475
Dodd, N.J.F. 1975. PHA and lymphocyte membrane fluidity.Nature (London) 257:827
Edelman, G.M. 1976. Surface modulation in cell recognition and growth; Some new hypotheses on phenotypic alteration and transmembranous control of cell surface receptors.Science 94:218
Esselman, W.J., Miller, H.C. 1974. Brain and thymus lipid inhibition of antibrain-associated θ-cytotoxicity.J. Exp. Med. 139:445
Farias, R.N., Bloj, B., Morero, R.D., Sineriz, F., Trucco, R.E. 1975. Regulation of allosteric membrane-bound enzymes through changes in membrane lipid composition.Biochim. Biophys. Acta 451:231
Feinstein, M.B., Fernandez, S.M., Sha'afi, R.I. 1975. Fluidity of natural membranes and phosphatidylserine and ganglioside dispersions. Effects of local anaesthetics, cholesterol and protein.Biochim. Biophys. Acta 413:354
Fisher, D.B., Mueller, G.C. 1971. Studies on the mechanism by which phytohaemagglutinin rapidly stimulates phospholipid metabolism of human lymphocytes.Biochim. Biophys. Acta 248:434
Gaffney, B.J. 1975. Fatty acid chain flexibility in the membranes of normal and transformed fibroblasts.Proc. Nat. Acad. Sci. USA 72:664
Gardas, A., Koscielak, J. 1973. New form of A-, B-, and H-blood-group-active substances extracted from erythrocyte membranes.Eur. J. Biochem. 32:178
Gardas, A., Koscielak. J. 1974. Megaloglycolipids — unusually complex glycosphingolipids of human erythrocyte membrane with A-, B-, H- and I-blood group specificity.FEBS. Lett. 42:101
Gottfried, E.L. 1972. Lipid patterns of leukocytes in health and disease.Semin. Hematol. 9:241
Gunther, G.R., Wang, J.L., Yahara, I., Cunningham, B.Y., Edelman, G.M. 1973. Concanavalin A derivatives with altered biological activities.Proc. Natl. Acad. Sci. USA. 70:1012
Heyningen, W.E. van 1974. Gangliosides as membrane receptors for tetanus toxin, cholera toxin and serotonin.Nature (London) 249:415
Hollenberg, M.D., Fishman, P.H., Bennett, V., Cuatrecasas, P. 1974. Cholera toxin and cell growth: Role of membrane gangliosides.Proc. Nat. Acad. Sci. USA 71:4224
Ji, T.H. 1974. Crosslinking of glycolipids in erythrocyte ghost membrane.J. Biol. Chem. 249:7841
Jost, P., Waggoner, A.S., Griffith, O.H. 1971. Spin labeling and membrane structure.In: The Structure and Function of Biological Membranes. L.I. Rothfield, editor. Ch. 3, p 84. Academic Press, New York
Keana, J.F.W., Keana, S.B., Beetham, D. 1967. A new versatile spin label.J. Am. Chem. Soc. 89:3055
Keith, A.D., Horvat, D., Snipes, W. 1974. Spectral characterization of 15N spin labels.Chem. Phys. Lipids 13:49
King, C.A., Heyningen, W.E. van 1973. Deactivation of cholera toxin by a sialidase-resistant monosialosylganglioside.J. Infect. Dis. 127:639
Kury, P.G., McConnell, H.M. 1975. Regulation of membrane flexibility in human erythrocytes.Biochemistry 14:2798
Kury, P.G., Ramwell, P.W., McConnell, H.M. 1974. The effect of prostaglandins E1 and E2 on the human erythrocyte as monitored by spin labels.Biochem. Biophys. Res. Commun. 56:478
Levy, H.B., Sober, H.A. 1960. A simple chromatographic method for the preparation of gamma globulin.Proc. Soc. Exp. Biol. Med. 103:250
Masuzawa, Y., Osawa, T., Inoue, K., Nojima, S. 1973. Effect of various mitogens on the phospholipid metabolism of human peripheral lymphocytes.Biochim. Biophys. Acta 326:339
Pantelouris, E.M. 1968. Absence of thymus in a mouse mutant.Nature (London) 217:370
Pascher, I. 1976. Molecular arrangements in sphingolipids. Conformation and hydrogen bonding of ceramide and their implication on membrane stability and permeability.Biochim. Biophys. Acta 455:433
Peters, J.H., Hausen, P. 1971. Effect of phytohaemagglutinin on lymphocyte membrane transport. II. Stimulation of ‘facilitated diffusion’ of 3-0-methyl-glucose.Eur. J. Biochem. 19:509
Quastel, M.R., Kaplan, J.G. 1970. Early stimulation of potassium uptake in lymphocytes treated with PHA.Exp. Cell Res. 63:230
Redwood, W.R., Polefka, T.G. 1976. Lectin-receptor interactions in liposomes. II. Interaction of wheat germ agglutinin with phosphatidyl choline vesicles containing incorporated monosialoganglioside.Biochim. Biophys. Acta 455:631
Révész, T., Greaves, M. 1975. Ligand-induced redistribution of lymphocyte membrane ganglioside GM1.Nature (London) 257:103
Sackman, E., Trauble, H., Galla, H., Overath, P. 1973. Lateral diffusion, protein mobility and phase transitions inEscherichia coli membranes. A spin label study.Biochemistry 12:5360
Sauerheber, R.D., Gordon, L.M., Crosland, R.D., Kuwahara, M.D. 1977. Spin label studies on rat liver and heart plasma membranes: Do probe-probe interactions interfere with the measurement of membrane properties?J. Membrane Biol. 31:131
Sharom, F.J., Barratt, D.G., Thede, A.E., Grant, C.W.M. 1976. Glycolipids in model membranes: Spin label and freeze etch studies.Biochim. Biophys. Acta 455:485
Slomiany, B.L., Slomiany, A. 1977. Complex glycosphingolipids with blood group A specificity.FEBS. Lett. 73:175
Toyoshima, S., Osawa, T. 1975. Lectins fromWistaria floribunda seeds and their effect on membrane fluidity of human peripheral lymphocytes.J. Biol. Chem. 250:1655
Verma, S.P., Wallach, D.F.H. 1975. Evidence for constrained lipid mobility in the erythrocyte ghost. A spin label study.Biochim. Biophys. Acta 382:73
Wedner, H.J., Parker, C.W. 1976. Lymphocyte activation.Prog. Allergy 20:195
Weiss, D.E. 1973a. Lipid mobility and function in biological membranes.Experientia 29:249
Weiss, D.E., 1973b. The role of lipid in energy transmission and conservation in functional biological membranes.Sub-Cell. Biochem. 2:201
Whitney, R.B., Sutherland, R.M. 1973. Effects of chelating agents on the interaction of phytohaemagglutinin with lymphocytes and the subsequent stimulation of amino acid uptake.Biochim. Biophys. Acta 298:790
Zenser, T.V., Petrella, V.J., Hughes, F. 1976. Spin-labeled stearates as probes for microenvironment of murine thymocyte adenylate cyclase-cyclic adenosine 3′∶5′-monophosphate system.J. Biol. Chem. 251:7431
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Curtain, C.C., Looney, F.D., Marchalonis, J.J. et al. Changes in lipid ordering and state of aggregation in lymphocyte plasma membranes after exposure to mitogens. J. Membrain Biol. 44, 211–232 (1978). https://doi.org/10.1007/BF01944222
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01944222