Abstract
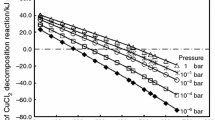
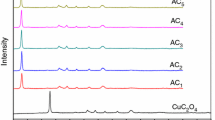
The thermal decomposition course of copper acetate monohydrate (CuAc) was examined on heating up to 600°C at various rates, by TG, DTA and DSC. Non-isothermal kinetic and thermodynamic parameters were determined in air or nitrogen. SEM was used to describe the decomposition course and the solid products were identified by IR and XRD analysis. The results indicated that CuAc was dehydrated at 190°C and then partially decomposed at 220°C, giving rise to CuO in addition to a minor portion of Cu2O and Cu4O3. The last two oxides seemed to facilitate the decomposition of the rest of the anhydrous acetate. Cu2O and Cu4O3 were oxidized in air at >400°C, in a process that did not occur in nitrogen.
Similar content being viewed by others
References
D. C. K. Lin and J. B. Westmore, Can. J. Chem., 51 (1973) 2999.
D. L. Trim, Design of Industrial Catalysts, Chemical Engineering Monographs 11, Elsevier, Amsterdam 1980.
M. E. Brown, D. Dollimore and A. K. Galwey, Reactions in the Solid State, Comprehensive Chemical Kinetics, Vol. 22, Elsevier, Amsterdam 1980.
M. I. Zaki and N. Sheppard, J. Catal., 80 (1983) 114.
N. H. Tannent and T. Baird, Stud. Conserv., 30 (1985) 73.
D. M. Griffiths and C. H. Rochester, J. Chem. Soc. Faraday Trans. 1, 74 (1978) 403.
S. A. A. Mansour, G. A. M. Hussein and M. I. Zaki, Rect. Solids, 8 (1990) 197.
S. A. A. Mansour, Thermochim. Acta, in press.
K. Nakamoto, Infrared Spectra of Inorganic and Coordination Compounds, Wiley, New York, 1970, pp. 220–224.
F. F. Bently, L. D. Smithson and A. L. Rozek, Infrared Spectra and Characteristics Frequencies ∼700-300 cm−1, Wiley, New York 1968, p. 1517–1518.
J. Mastowska and Baranowska, J. Thermal Anal., 29 (1984) 309.
M. D. Judd, B. A. Plunkett and M. I. Pope, J. Thermal Anal., 6 (1974) 555.
K. C. Path, G. V. Chandrashekhar, M. V. George and C. N. R. Rao, Canad J. Chem., 46 (1968) 257.
T. Ozawa, J. Thermal Anal., 2 (1970) 301.
Author information
Authors and Affiliations
Additional information
It is a pleasure to thank the Queen's University of Belfast, particularly the staff of the Electron Microscope Unit for assistance in obtaining the electron micrographs. Thanks are also due to the Egyptian Government for the granted fellowship.
Rights and permissions
About this article
Cite this article
Mansour, S.A.A. Thermoanalytical investigations of the decomposition course of copper oxysalts. Journal of Thermal Analysis 46, 263–274 (1996). https://doi.org/10.1007/BF01979966
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01979966