Abstract
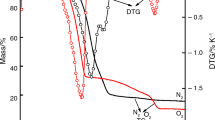
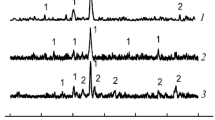
Thermal decomposition of pure Fe(OH)3 and mixed with Co(OH)2 were studied using TG, DTA, kinetics of isothermal decomposition and electrical conductivity measurements. The thermal products were characterized by X-ray diffraction and IR spectroscopy. The TG and DTA analysis revealed the presence of Co2+ retards the decomposition of ferric hydroxide and the formation of α-Fe2O3. The kinetics of decomposition showed that the mixed samples need higher energy to achieve thermolysis. The investigation of thermal products of mixed samples indicated the formation of cobalt ferrite on addition ofx=1 or 1.5 cobalt hydroxide. The electrical conductivity accompanying the thermal decomposition decreases in presence of low ratio of Co2+ (x=0.2) via the consumption of holes created during thermal analysis. The continuous increase in σ values on increasing of Co2+ concentration corresponded to the electron hopping between Fe2+ and Co3+.
Zusammenfassung
Mittels TG, DTA und der Kinetik von Messungen der isothermen Zersetzung und der elektrischen Leitfähigkeit wurde die Zersetzung von Fe(OH)3 in reinem Zustand und vermengt mit Co(OH)2 untersucht. Die thermischen Produkte wurden mittels Röntgendiffraktion und IR-Spektroskopie charakterisiert. TG und DTA zeigen, daß die Zersetzung von Eisen(III)-hydroxid und die Bildung von -Fe2O3 durch Gegenwart von Co2+ verzögert wird. Die Zersetzungskinetik zeigt, daß die Mischproben mehr Energie für die Thermolyse benötigen. Die Untersuchung der thermischen Produkte zeigt die Bildung von Cobaltferrit bei Zusatz vonx=1 oder 1,5 Cobalthydroxid. Die elektrische Leitfähigkeit nimmt bei der thermischen Zersetzung in Gegenwart von niedrigen Co2+-Konzentrationen (x=0.2) durch Verbrauch der bei der Thermoanalyse geschaffenen Löcher ab. Das monotone Ansteigen der -Werte bei steigender Co2+-Konzentration stimmt mit dem Überspringen von Elektronen zwischen Fe2+ und Co3+ überein.
Similar content being viewed by others
References
V. V. Boldyrev, M. Bulens and B. Delmun, The control of the reactivity of solids, Elsevier, Amsterdam 1979.
B. L. Kuglar and J. W. Gryder, J. Catal., 44 (1979) 126.
T. Uenatsu, K. Inamura, K. Hirari and H. Hashimoto, J. Catal., 45 (1976) 68.
A. C. C. Tseung and J. R. Goldstein, J. Mat. Sci., 7 (1972) 1383.
R. R. Jaran and P. A. Sermon, J. Chem. Soc. Faraday Trans. I, 81 (1985) 2577.
A. A. Said, E. A. Hassan and K. M. Abd El-Salaam, Surf. Technol., 20 (1983) 123.
J. V. Smith, Ed., X-ray Powder Data File, American Soc. for Testing Materials, Philadelphia 1960.
C. Barriga, P. Lavela, J. Morales and J. Tirado, J. Coll. Interface Sci., 138 (1990) 565.
B. Gillot, F. Bouton, F. Chassagneux and A. Rousset, J. Solid State Chem., 33 (1980) 245.
B. Gillot, R. M. Benloucif and A. Rousset, ibid., 39 (1981) 329.
B. Gillot, Mater. Res. Bull., 13 (1978) 783.
B. Gillot, A. Rousset and G. Dupre, ibid., 25 (1978) 263.
B. Gillot, F. Jemmal and A. Rousset, J. Solid State Chem., 50 (1983) 138.
B. Gillot, R. M. Benloucif and F. Jemmal, J. Mat. Sci., 19 (1984) 3806.
H. Takai and S. Shiba, J. Phys. Soc. Jpn., 21 (1966) 1255.
M. Figlarz, J. Guenot and J. N. Tournemolle, J. Mat. Sci., 19 (1974) 772.
J. T. Richardson and L. W. Vernson, J. Phys. Chem., 62 (1958) 1153.
H. De Bie and P. Doyen, Cobalt, 15 (1962) 3.
G. A. El-Shobaky, N. Ghoneim and I. F. Hewaidy, Thermochim. Acta, 5 (1982) 105.
R. A. Nyquist and R. O. Kagel, Infrared Spectra of Inorganic Compounds, Academic Press, New York and London 1971.
G. A. El-Shobaky, N. Ghoneim, I. F. Hewaidy and I. Morsi, Thermochim. Acta, 61 (1983) 107.
J. M. Jimenez-Mateos, J. Morales and J. L. Tirado, React. Solids, 7 (1989) 235.
B. Gillot and A. Rousset, J. Solid State Chem., 65 (1986) 322.
J. R. Goldstein and C. C. Tseung, J. Catal., 32 (1974) 33.
R. D. Waldron, Phys. Rev., 99 (1955) 1727.
H. T. S. Britton, S. J. Gregg and G. W. Winsor, Trans. Faraday Soc., 48 (1952) 63.
B. Gillot, R. M. Benloucif and A. Rousset, Phys. Status Solid (a), 65 (1981) 205.
F. Chassagneux and A. Rousset, J. Solid State Chem., 16 (1976) 161.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Said, A.A., Abd El-Salaam, K.M., Hassan, E.A. et al. A study on the thermal decomposition of iron-cobalt mixed hydroxides. Journal of Thermal Analysis 39, 309–321 (1993). https://doi.org/10.1007/BF01983530
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01983530