Abstract
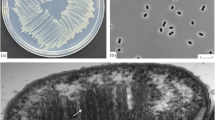
Enrichment cultures containing marine plankton from oxygenated coastal waters (50–108% saturated) with supersaturated levels of methane (>700% saturated) yielded a strictly anaerobic methanogenic bacterium. Nonmotile, non-spore-forming, regular to slightly irregular cocci (0.5–0.8µm) were evident by phase contrast, epifluorescence, and scanning electron microscopies. The unpurified isolate required NaCl for growth, with maximal methanogenesis at 240 mM NaCl at 22°C. The optimal temperature range for growth was 22–31°C, and the optimal range for methanogenesis was 26–35°C. Mono-, di-, and trimethylated amines or methanol were substrates for methanogenesis; sodium acetate and H2:CO2 were not. The DNA base composition was 42 ±1% guanine plus cytosine. Serology suggested the isolate may be a new strain ofMethanococcoides methylutens. Morphology, growth physiology, DNA base content, and serology are all consistent with the type description ofM. methylutens, a methylotrophic methanogen isolated from submarine sediments.
Similar content being viewed by others
Literature Cited
Alldredge AL, Cohen Y (1987) Can microscale chemical patches persist in the sea? Microelectrode study of marine snow, fecal pellets. Science 235:689–691
Balch WE, Wolfe RS (1976) New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth ofMethanobacterium runinantium in a pressurized atmosphere. Appl Environ Microbiol 32:781–791
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: reevaluation of a unique biological group. Microbiol Rev 43:260–296
Beers JR (1967) The species distribution of some naturally occurring quaternary ammonium compounds. Comp Biochem Physiol 21:11–21
Blake DR, Mayer EW, Tyler SC, Makiye Y, Monaque DC, Rowland FS (1982) Global increase in atmospheric methane concentrations between 1978 and 1980. Geophys Res Lett 9:477–480
Blunden G, Gordon SM, McLean WFH, Guiry MD (1982) The distribution and possible taxonomic significance of quaternary ammonium and other Dragendorff-positive compounds in some genera of marine algae. Bot Mar 25:563–567
Brooks JM, Sackett WM (1973) Sources, sinks and concentrations of light hydrocarbons in the Gulf of Mexico. J Geophys Res 78:1069–1071
Brooks JM, Reid DF, Bernard BB (1981) Methane in the upper water column of the Northwestern Gulf of Mexico. J Geophys Res 86:11029–11040
Burke RA, Reid DF, Brooks JM, Lavoie DM (1983) Upper water column methane geochemistry in the eastern tropical North Pacific. Limnol Oceanogr 28:19–32
Scripps Institution of Oceanography (1987) Physical, chemical, and biological data report, CalCOFI cruise 8609, CalCOFI 8611. University of California, San Diego: SIO Ref 87-7
Scripps Institution of Oceanography (1987) Physical, chemical, and biological data report, CalCOFI cruise 8703, CalCOFI 8705. University of California, San Diego: SIO Ref 87-19
Scripps Institution of Oceanography (1988) Physical, chemical, and biological data report, CalCOFI cruise 8709, CalCOFI 8711. University of California, San Diego: SIO Ref 88-8
Scripps Institution of Oceanography (1989) Physical, chemical, and biological data report, CalCOFI cruise 8801, CalCOFI 8805. University of California, San Diego: SIO Ref 88-23
Scripps Institution of Oceanography (1989) Physical, chemical, and biological data report, CalCOFI cruise 8901, CalCOFI 8904. University of California, San Diego: SIO Ref 89-26
Carpenter JH (1965) The Chesapeake Bay Institute technique for the Winkler dissolved oxygen method. Limnol Oceanogr 10:141–143
Cheeseman P, Tosm-Wood A, Wolfe RS (1972) Isolation and properties of a fluorescent compound, factor 420, fromMethanobacterium strain M.O.H. J Bacteriol 112:527–531
Conrad R, Seiler W (1988) Methane and hydrogen in seawater (Atlantic Ocean). Deep-Sea Res 35:1903–1917
Conway de Macario E, Macario AJL, Jovell RJ (1986) Slide immunoenzymatic assay (SIA) in hybridoma technology. Methods Enzymol 121:509–525
Doodema HJ, Vogels GD (1978) Improved identification of methanogenic bacteria by fluorescence microscopy. Appl Environ Microbiol 36:752–754
Dubach AC, Bachofen R (1985) Methanogens: a short taxonomic review. Experientia 41:441–445
Fraser PJ, Khalil MAK, Rasmussen RA, Crawford AJ (1981) Trends of atmospheric methane in the southern hemisphere. Geophys Res Lett 8:1063–1066
Goldman JC (1984) Conceptual role for microaggregates in pelagic waters. Bull Mar Science, 35:462–476
Heijthuijsen JHFG, Hansen TA (1989) Betaine fermentation and oxidation by marineDesulfuromonas strains. Appl Environ Microbiol 55:965–969
Hippe H, Caspai D, Fiebig K, Gottschalk G (1979) Utilization of trimethylamine and other N-methyl compounds for growth and methane formation byMethanosarcina barkeri. Proc Natl Acad Sci USA 76:494–498
Joergensen BB, Revsbech NP (1985) Diffusive boundary layers and oxygen uptake of sediment and detritus. Limnol Oceanogr 30:111–122
Johnson JL (1981) Genetic characterization. In: Gerhardt P (ed) Manual of methods for general bacteriology. Washington, DC: American Society for Microbiology, pp 450–472
Khalil M, Rasmussen RA (1990) Atmospheric methane: recent global trends. Environ Sci & Technol 24:549–553
Kiener A, Leisinger T (1983) Oxygen sensitivity of methanogenic bacteria. Sys Appl Microbiol 4:305–312
King GM (1984) Utilization of hydrogen, acetate and “non-competitive” substrates by methanogenic bacteria in marine sediments. Geomicrobiol J, 3:275–306
King GM (1988) Methanogenesis from methylated amines in a hypersaline algal mat. Appl Environ Microbiol 54:130–136
Kubitschek HE (1958) Electronic counting and sizing of bacteria. Nature 182:234–235
Lamontagne RA, Swinnerton JW, Linnenbom VJ (1974) C1–C4 hydrocarbons in the North and South Pacific. Tellus, 26:71–77
Lashof DA, Ahuja DR (1990) Relative contribution of green-house gas emission to global warming. Nature 344:529–531
Macario AJL, Conway de Macario E (1983) Antigenic fingerprinting of methanogenic bacteria with polyclonal antibody probes. Syst Appl Microbiol 4:451–458
Macario AJ, Conway de Macario E (1985) Monoclonal antibodies of predefined molecular specificity for identification and classification of methanogens and for probing their ecologic niches. In: Macario AJ, Conway de Macario E (eds) Monoclonal antibodies against bacteria. Orlando: Academic Press, pp 213–247
Macario AJL, Conay de Macario E (1986) Methanogens in digestors: identification and quantification in microsamples by direct testing with antibody probes. In: Institute of Gas Technology (ed), Energy from biomass and wastes X. Essex: Elsevier Press, pp 1009–1020
Macario AJL, Conway de Macario E (1988) Quantitative immunologic analysis of the methanogenic fluora of digestors reveals a considerable diversity. Appl Environ Microbiol 54:79–86
Mah RA, Smith MR (1981) The methanogenic bacteria. In: Starr MP, Stolp H, Truper HG, Balows A, Schlegel HG (eds) The prokaryotes. New York: Springer-Verlag, pp 848–977
Möller B, Oßmer R, Howard BH, Gottschalk G, and Hippe H (1984)Sporomusa, a new genus of gram-negative anaerobic bacteria includingSporomusa sphaeroides spec. nov. andSporomusa ovata, spec. nov. Arch Microbiol 139:388–396
Naumann E, Hippe H, Gottchalk G (1983) Betaine; New oxidant in the Stickland reaction and methanogenesis from betaine andl-alanine by aClostridium sporogenes-Methanosarcina barkeri coculture. Appl Environ Microbiol 45:474–483
Oremland RS (1979) Methanogenic activity in plankton samples and fish intestines: a mechanism for in situ methanogenesis in oceanic surface waters. Limnol. Oceanogr 24:1136–1141
Oremland RS (1988) Biogeochemistry of methanogenic bacteria. In: Zehnder AJB (ed) Biology of anaerobic microorganisms. New York: Wiley and Sons, pp 641–705
Paerl HW, Carlton RG (1988) Control of nitrogen fixation by oxygen depletion in surface-associated microzones. Nature 332:260–262
Paerl HW, Prufert LE (1987) Oxygen-poor microzones as potential sites of microbial N2 fixation in nitrogen-depleted aerobic marine waters. Appl Environ Microbiol 53:1078–1087
Pfennig N, Wagener S (1986) An improved method of preparing wet mounts for photomicrographs of microorganisms. J Microbiol Methods, 4:303–306
Rasmussen RA, Khalil, MAK (1981) Increase in the concentration of atmospheric methane. Atmos Environ 15:883–886
Rudd JWM, and Taylor CD (1980) Methane cycling in aquatic environments. Adv Aquatic Microbiol 2:77–150
Scranton MI, Brewer PG (1977) Occurrence of methane in the near-surface waters of the western subtropical North-Atlantic. Deep-Sea Res 24:127–138
Sieburth JM (1983) Microbiological and organic-chemical processes in the surface and mixed layers. In: Liss PS, Slinn WGN (eds) Air-sea exchange of gases and particles. D. Reidel Publishing, pp 121–172
Sieburth JM (1987) Contrary habitats for redox-specific processes: methanogenesis in oxic waters and oxidation in anoxic waters. In: Sleigh MA (ed) Microbes in the sea. Chichester, England: Halsted Press, pp 11–38
Sowers KR, Ferry JG (1983) Isolation and characterization of a methylotrophic marine methanogen,Methanococcoides methylutens gen. nov., sp. nov. Appl Environ Microbiol, 45:684–690
Swinnerton JW, Linnenbom VJ, Cheek CH (1969) Distribution of methane and carbon monoxide between the atmosphere and natural waters. Environ Sci Tech 3:836–838
Traganza ED, Swinnerton JW, Cheek CH (1979) Methane supersaturation and ATP-zooplankton blooms in near-surface waters of the Western Mediterranean and the subtropical North Atlantic Ocean. Deep-Sea Res 26A:1237–1245
UNESCO (1981) Background papers and supporting data on the Practical Salinity Scale, 1978. UNESCO Technical Papers in Marine Science, Paris, France
Weiss RF (1970) The solubility of nitrogen, oxygen and argon in water and seawater. Deep-Sea Res 17:721–735
Whitman WB (1985) Methanogenic bacteria. In: Woese CR, Wolfe RS (ed) The bacteria. Orlando: Academic, pp 3–84
Wiesenburg DA, Guinasso NLJ (1979) Equilibrium solubilities of methane, carbon monoxide, and hydrogen in water and sea water. J Chem Eng Data, 24:356–360
Wolfe RS (1971) Microbial formation of methane. In: Rose AH, Wilkinson JF (ed) Advances in microbial physiology. New York: Academic Press, pp 107–146
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cynar, F.J., Yayanos, A.A. Enrichment and characterization of a methanogenic bacterium from the oxic upper layer of the ocean. Current Microbiology 23, 89–96 (1991). https://doi.org/10.1007/BF02092256
Issue Date:
DOI: https://doi.org/10.1007/BF02092256