Abstract
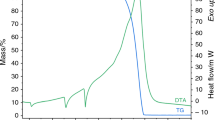
The thermal decompositions of solid complexes of the type Ni(NCS)2L2 (L = pyridine,α-picoline,β-picoline, 2,6-lutidine, and quinoline) were studied by means of the derivatograph. It was found that the decompositions of complexes with pyridine,α-picoline, 2,6-lutidine, and quinoline (the pseudo-octahedral complex) are onestep processes, and those of complexes withβ-picoline and quinoline (the squareplanar complex) consist of two steps.
Diffuse reflectance spectra were recorded to elucidate the structures of the decomposition intermediates.
The reasons for the different stoichiometries of decomposition for complexes of the same type are discussed.
Résumé
Etude de la décomposition thermique des complexes solides de formule générale Ni(SCN)2L2 (L=pyridine,α-picoline,β-picoline, lutidine-2,6 et quinoléine) à l'aide du “Derivatograph”. La décomposition des complexes de la pyridine, l'α-picoline, la lutidine2, 6 et la quinoléine (complexes pseudo-octaédriques) se déroule en une seule étape, tandis que celle des complexes de laβ-picoline et de la quinoléine (complexes plans carrés) s'effectue en deux temps. Emploi des spectres de réflexion diffuse pour élucider la structure des produits intermédiaires de la décomposition. Interprétation des différences de stoechiométrie pendant la décomposition des complexes du même type.
Zusammenfassung
Die festen Komplexverbindungen Ni(NCS)2L2 (L=Pyridin,α- undβ-Picolin, 2,6-Lutidin und Chinolin) wurden derivatographisch untersucht. Die Komplexe mit Pyridin,α-Picolin, 2,6-Lutidin und Chinolin (pseudooktaedrische.Komplexe) zerfallen in einer Stufe, die entsprechenden Verbindungen mitβ-Picolin und Chinolin (quadratisch-planare Komplexe) in zwei Stufen. Die Strukturbestimmung der intermediären Zersetzungsprodukte wurde mit Hilfe der diffusen Reflexionsspektren durchgeführt. Die Ursachen der verschiedenen Zersetzungsstöchiometrien der Komplexe von gleichem ⌈yp wurden diskutiert.
Резюме
С помощью дериватогр афа изучен терморасп ад твердофазных компле ксов типа Ni(NCS)2L2 (L=пиридин, αа-пи колин, β-пиколин, 2,6-лути дин и хинолин). Установлено, что распад комплексо в с пиридином, αа-пикол ином, 2,6-лутидином и хинолино м (псевдо-октаэдрическ ий комплекс) является одноступенчатым про цессом, а распад компл ексов с β-пиколином и хиноли ном (планарный компле кс) —двухступенчатым. Для выяснения структуры промежуто чных продуктов распа да измерен спектр диффузионног о отражения. Обсуждает ся причина различной стехиометрии распад а в случае комплексoв аналогичного типа.
Similar content being viewed by others
References
F. Basolo andR. G. Pearson, “Mechanisms of Inorganic Reactions”, 2nd Edition, J. Wiley, New York, 1967, p. 256.
C. H. Langford andH. B. Gray, Ligand Substitution Processes, W. A. Benjamin, New York, 1965; Russian translation: “Procesy zamescenija ligandov”, Izdat. Mír, Moscow 1969, p. 123.
E. Jóna, T. Šramko andJ. Gažo, Chem. Zvesti, 22 (1968) 648.
E. Jóna, V. Jesenák andT. Šramko, Chem. Zvesti, 23 (1969) 420.
T.Šramko, E.Jóna and A.Sirota, Proceedings of the 2nd Conference on Coordination Chemistry, Smolenice-Bratislava, 1969, p. 239.
E. Jóna, T. Šramko, K. Kohout, A. Sirota andJ. Gažo, Chem. Zvesti, 25 (1971) 241.
H. C. A.King, E.Körös and S. M.Nelson, J. Chem. Soc., (1963) 5443.
S. M. Nelson, T. M. Shepherd, Inorg. Chem., 4 (1965) 813.
M. A. Poraj-Koshic, Zh. Neorg. Khim., 4 (1959) 730.
A. V. Logan andD. W. Carle, J. Amer. Chem. Soc., 74 (1952) 5224.
F. Paulik, J. Paulik andL. Erdey, Z. anal. Chem., 160 (1958) 241.
W. W. Wendlandt andJ. P. Smith, The Thermal Properties of Transition Metal Ammine Complexes, Elsevier, Amsterdam, 1967, p. 158.
L. G. Berg, Vvedenije v termografiu, Izdat. Nauka, Moscow, 1969, p. 125.
W. W. Wendlandt, Chemist-Analyst, 53 (1964) 71.
D. H. Brown, R. N. Nuttall andD. W. A. Sharp, J. Inorg. Nucl. Chem., 26 (1964) 1151.
A. V. Downs andD. A. Ongley, Chem. and Ind., 23 (1963) 493.
C. D.Flint and M.Goodgame, J. Chem. Soc., A (1970) 442.
G. Maki, J. Chem. Phys., 29 (1958) 162.
M. Ciampolini, Inorg. Chem., 5 (1966) 35.
M. Ciampolini andN. Nardi, Inorg. Chem., 6 (1967) 445.
L. Erdey, G. Liptay, Periodica Polytechnica, 7 (1963) 223.
G Liptay, K. Burger, E. Papp-Molnár, Sz. Szebeni andF. Ruff, J. Inorg. Nucl. Chem., 31 (1969) 2359.
W. W. Wendlandt andS. I. Ali, Z. anorg. allg. Chem. 337 (1965) 6.
P. B. Bowman, L. B. Rogers, J. Inorg. Nucl. Chem., 28 (1966) 2215.
W. Kemula andJ. Czarnecki, Roczn. chim., 41 (1967) 1463.
A. K. Majumdar, A. K. Mukherjee, A. K. Mukherjee, J. Inorg. Nucl. Chem., 26 (1964) 2177.
G. Liptay, Kémiai Közlemények, 32 (1969) 389.
M. A. Poraj-Koshic andA. C. Ancyshkina, Z. Kristallogr., 3 (1958) 686.
D. Young, Decomposition of Solids, Pergamon Press, 1966. Russian translation: Kinetika razloženija tverdych veščestv, Izdat. Mir, Moscow, 1969, p. 97.
S. Škramovsky, R. Förster andG. F. Hüttig, Z. Phys. Chem. B, 25 (1934) 1.
N. Hurduc, L. Odochian, Y. A. Schneider andE. Segal, Rev. Roum. Chim., 11 (1966) 1453.
R. J. H. Clark andC. S. Williams, Spectrochim. Acta, 2 (1966) 1081.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jóna, E., Šramko, T. & Gažo, J. Heterogeneous reactions of solid nickel(II) complexes, III. Journal of Thermal Analysis 4, 61–71 (1972). https://doi.org/10.1007/BF02100951
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02100951