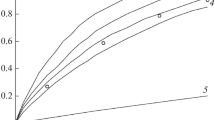
Abstract
Conditions are presented for application of the Piloyan method to the DTA of poly-nitro aromatic compounds. Activation energies (E) of the thermal decomposition and the initial valuesT D of the exotherms are determined for trinitrotoluene, trinitro-m-xylene, trinitromesitylene, picryl chloride and dichlorotrinitrobenzene. Linear relationships are derived between the termsE · T −D and published kinetic data on these compounds, obtained by an isothermal manometric method. The mechanisms of the primary steps in the thermolyses of these polynitro compounds are discussed. A negative influence on their thermal stability has been confirmed, arising from the contact of the measured compounds with the glass surface.
Résumé
On présente les conditions de l'application de la méthode Piloyan à l'ATD des composés polynitroaromatiques. On a déterminé les énergies d'activation (E) et les valeurs initialesT D des réactions exothermiques pour le trinitrotoluène, le trinitro-m-xylène, le trinitromé sitylène, le chlorure picrylique et le dichloro-trinitrobenzène. On a trouvé des corrélations linéaires entre les valeursE.T −D et les données cinétiques publiées des composés mentionnés obtenues par une méthode isotherme manométrique. On discute le mécanisme de l'étape primaire de la thermolyse des composés polynitrés étudiés. On a confirmé l'influence négative, sur la stabilité thermique, provenant du contact de ces composés avec le verre.
Zusammenfassung
Die Bedingungen der Anwendung der Methode von Piloyan bei der DTA polynitroaromatischer Verbindungen werden beschrieben. Die Aktivierungsenergien (E) der thermischen Zersetzung und die AnfangswerteT D der exothermen VorgÄnge werden für Trinitrotoluol, Trinitro-m-xylol, Trinitromesitylol, Picrylchlorid und Dichlortrinitro-benzol bestimmt. Lineare ZusammenhÄnge werden zwischen den WertenE.T −D und den unter Anwendung der isothermen manometrischen Methode erhaltenen veröffentlichten kinetischen Angaben der besagten Verbindungen, abgeleitet. Der Mechanismus der primÄren Stufe in der Thermolyse der untersuchten Polynitroverbindungen wird diskutiert. Der sich aus dem Kontakt der gemessenen Verbindungen mit dem Glas ergebende negative Einflu\ auf ihre StabilitÄt wird bestÄtigt.
РЕжУМЕ
пРЕДстАВлЕНы УслОВИ ь пРИМЕНЕНИь МЕтОДА пИлОьНА Дль ДтА пОлИН ИтРОАРОМА-тИЧЕскИх сОЕДИНЕНИИ. ОпРЕДЕлЕ Ны ЁНЕРгИИ АктИВАцИИ (E) тЕРМИЧЕскОгО РАжлОж ЕНИь И НАЧАлАT D ЁкжОтЕРМ Дль тРИНИтРОтОлУОлА, тРИНИтРО-М-ксИлОлА, тР ИНИтРОМЕжИтИлА, пИк-Р ИлхлОРИДА И тРИхлОРтРИНИтРОБЕ НжОлА. УстАНОВлЕНА лИНЕИНАь ВжАИМОсВьж ь МЕжДУ ВЕлИ-ЧИНАМИ E · т −1D И кИНЕтИЧЕскИМИ ДАННыМИ, ОпУБлИкОВАННыМИ И кО тОРыЕ БылИ пОлУЧЕНы ИжОтЕРМИЧЕскИМ МАНО МЕтРИЧЕскИМ МЕтОДОМ. ОБсУжДЕН МЕхАНИжМ пЕРВОНАЧАл ьНОИ стАцИИ тЕРМОлИжА ИсслЕДОВА ННых пОлИНИтРОАРОМА тИЧЕскИх сОЕДИНЕНИИ. ВОжНИкАУ ЩИИ ВслЕДстВИИ кОНтАктА ИжУЧЕННых с ОЕДИНЕНИИ с пОВЕРхНО стьУ стЕклА ЁФФЕкт ОкАжыВАЕт ОтР ИцАтЕльНОЕ ВлИьНИЕ НА тЕРМИЧЕск УУ стАБИльНОсть сОЕД ИНЕНИИ.
Similar content being viewed by others
References
B. S.Svetlov, Khim. Tekhnol., (1958) 422.
K. K. Andreev andA. F. Belyaev, Teoriya vzryvchatykh veschestv. (Theory of blasting materials) Gos. nauchnotekhn. izdat. Oborongiz, Moscow, 1960.
K. K. Andreev, Termicheskoye razlozheniye i goreniye vryvchatykh veschestv. (Thermal decomposition and combustion of blasting materials), Izdat. Nauka, Moscow, 1966.
Yu. Ya. Maksimov andV. A. Koroban, Teoriya vzryvchatykh veschestv. (Theory of blasting materials) Izdat. Vyschaya schkola, Moscow, 1967, p. 92.
R. N.Rogers, Jahrstag., Inst. Chem. Treib-Explosivstoffe Fraunhofer-Ges. 1971 (Publ. 1972) p. 41.
G. Krien, Differential Thermal Analysis ed. R. C. Mackenzie, Vol. 2. Academic Press, London, 1972, p. 353.
S. Takeyama andT. Yoshida, Kogyo Kayaku 36 (1975) 238.
L. W. Collins andL. D. Shaws, Thermochim. Acta, 21 (1977) 1.
Y. Hara, S. Kamei andH. Osada, Kogyo Kayaku, 34 (1973) 147.
Y. Hara, S. Kamei andH. Osada, Kogyo Kayaku, 34 (1973) 253.
Y. Hara andH. Osada, Kogyo Kayaku, 34 (1973) 343.
E. Kitajima, T. Hayakawa, T. Hashizume, S. Akihisa, Y. Hara andH. Osada, Kogyo Kayaku, 35 (1974) 22.
Y. Hara andH. Osada, Kogyo Kayaku, 35 (1974) 26.
Y. Hara, H. Eda andH. Osada, Kogyo Kayaku, 36 (1975) 66.
Y. Hara, H. Eda andH. Osada, Kogyo Kayaku, 36 (1975) 250.
Y. Hara, H. Eda andH. Osada, Kogyo Kayaku, 36 (1975) 255.
Y. Hara andH. Osada, Kogyo Kayaku, 37 (1976) 233.
Y. Hara, F. Kawano andH. Osada, Kogyo Kayaku, 38 (1977) 266.
Y. Hara, H. Matsubara andH. Osada, Kogyo Kayaku, 38 (1977) 338.
J. šesták andG. Bergen, Chem. Listy, 64 (1970) 695.
A. BlaŽek, Termická analýza (Thermal analysis). SNTL Prague, 1972.
S.Zeman and E.Zemanová (to be published).
H. E. Kissinger, J. Res. Nat. Bur. Std., 57 (1956) 217.
H. E. Kissinger, Anal. Chem., 29 (1957) 1702.
W. W. Wendlandt, Thermal Methods of Analysis. Wiley, New York, 1974.
W. W. Wendlandt, Termicheskiye metody analiza. (Thermal Methods of Analysis). Izdat. Mir, Moscow, 1978, p. 200.
R. L. Reed, L. Weber andB. S. Gottfried, J. & E. C. Fundamentals 4 (1965) 38.
R. Melling, F. W. Wilburn andR. M. McItosh, Anal. Chem., 41 (1969) 1275.
G. O. Piloyan, I. D. Ryabchikov andO. S. Novikova, Nature, 212 (1966) 1229.
S. Zeman, Thermostable Polynitroaromatic Compounds. Part I. Ph. D. Thesis, Univ. Chem. Technol., Pardubice, June 1973.
S. Zeman, Thermostable Polynitroaromatic Compounds. Part II. Sci. report PO 2-79. ÚŘad pro vyná lezy a objevy, Prague, Jan. 1979.
S. Zeman, Some Problems of Production of Dinitrosopentamethylenetetramine. Part I. Res. report 7503863, UVTEI-STK, Prague, 1975.
A. Tall, The Stability of Chempor. Final res. report 7605465, UVTEI-STK, Prague, 1976.
G. O. Piloyan, Vvedeniye v teoriyu termicheskogo analiza. (Introduction in the theory of thermal analysis). Izdat. Nauka, Moscow, 1967, p. 134.
K. K. Andreev, Teoriya vzryvchatykh veschestv. (Theory of blasting materials). Izdat Oborongiz, Moscow, 1963.
K. K. Andreev, Teoriya vzryvchatykh veschestv. (Theory of blasting materials). Izdat Vyschaya schkola, Moscow, 1967.
V. F. Sapranovich, Yu. Ya. Maksimov andM. E. Makrelova, Trudy MCHTI im. Mendeleeva, vyp. 75 (1973) 147.
Yu. Ya. Maksimov, N. V. Polyakov andV. F. Sapranovich, Trudy MCHTI im. Mendeleeva, vyp. 83 (1974) 55.
E.Hauseler, Symp. Chem. Problems Connected Stab. Explos., Stockholm, (1967) 34.
E. G. Janzen, J. Am. Chem. Soc., 87 (1965) 3531.
R. N. Rogers, Anal. Chem., 39 (1967) 730.
J. M. Rosen andJ. C. Dacons, Explosivstoffe, 16 (1968) 250.
J. C. Dacons, H. G. Adolph andM. J. Kamlet, J. Phys. Chem., 74 (1970) 3035.
H. J. Pasman, Th. M. Groothuizen andC. M. Vermeulen, Explosivstoffe, 17 (1969) 151.
Yu. Ya. Maksimov, Zh. Fiz. Khim., 45 (1971) 793.
Yu. Ya. Maksimov, Zh. Fiz. Khim., 46 (1972) 1726.
N. V. Polyakov, V. F. Sapranovich andYu. Maksimov, Trudy MCHTI im. Mendeleeva, vyp., 83 (1974) 51.
Yu. Ya. Maksimov andL. T. Pavlik, Zh. Fiz. Khim., 49 (1975) 649.
E. K. Fields andS. Meyerson, J. Org. Chem., 33 (1968) 4487.
G. Krien, Explosivstoffe, 13 (1965) 205.
J. W. Beckmann, J. S. Wilkes andR. R. Moguire, Thermochim. Acta, 19 (1977) 111.
S. A. Shackelford, J. W. Beckmann andJ. S. Wilkes, J. Org. Chem., 42 (1977) 4201.
R. N. Rogers, Thermochim. Acta, 11 (1975) 131.
J. Harris, Thermochim. Acta, 14 (1976) 183.
A. E. Simchen, Israel J. Technol., 7 (1969) 445.
M.Marcin, unpublished work, Chemko, StráŽske, 1971.
G. P. Sharnin, B. I. Buzykin, V. V. Nurgatin andI. E. Moysak, Zh. Org. Khim., 3 (1966) 82.
S. Zeman, Thermochim. Acta, 31 (1979) 269.
J. R. Holden andC. Dickinson, J. Phys. Chem., 73 (1969) 1203.
J. R. Holden andC. Dickinson, J. Phys. Chem., 71 (1967) 1129.
J. H. Bryden, Acta Cryst., Ser. B, 28 (1972) 1395.
A. G.Turovec and V. I.Danilova, Izv. Vysshykh Uchebn. Zavedent., Fizika, (1973) 68.
Author information
Authors and Affiliations
Additional information
The author is obliged to Mrs Klara Kováčová M. S. for her most generous help in processing the results of measurements on the Wang 2200 computer.
Rights and permissions
About this article
Cite this article
Zeman, S. Possibilities of applying the Piloyan method of determination of decomposition activation energies in the differential thermal analysis of polynitroaromatic compounds and of their derivatives. Journal of Thermal Analysis 17, 19–29 (1979). https://doi.org/10.1007/BF02156593
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02156593