Summary
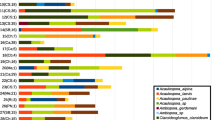
Genotypes of pearl millet (Pennisetum americanum L. Leeke) were examined for differences in vesicular-arbuscular mycorrhizal (VAM) colonization and response to inoculation. For thirty genotypes tested across three field locations there was a range of mycorrhizal colonization intensity between 25 and 56%. In another experiment with two male-sterile lines, restorer lines and their derived crosses, grown in pots filled with non-sterilized soil there were significant differences between genotypes for colonization by mycorrhiza. This showed hostgenotype dependence for mycorrhizal colonization.
Root growth rates, mycorrhizal root length, percentage root colonization and plant growth and P uptake were studied in ten genotypes. A set of 3 genotypes with similar root lengths varied significantly with regard to mycorrhizal root length and the percentage colonization. This supports the suggestion that VAM colonization and spread is dependent on the host genotype. The growth responses differed significantly between the genotypes and they also differed in their responses to P uptake and VAM inoculation. The utility of host-genotype dependent differences in VAM symbiosis in plant breeding is discussed.
Similar content being viewed by others
References
Azcon R and Ocampo N A 1980 Factors affecting the vesicular-arbuscular infection and mycorrhizal dependency of thirteen wheat cultivars. New Phytol. 87, 677–685.
Bertheau Y, Gianinazzi-Pearson V and Gianinazzi S 1980 Development et expression de l'association endomycorrhizienne chez le ble I. Mise en evidence d'un effet varietal. ann. Amelior. Plantes. 30, 67–78.
Bremner J M 1965 Total Nitrogen.In Methods of Soil Analysis (Part 2). Ed. C A Black, Amer. Soc. Agron. Madison, Wisc. pp 1149–1179.
Buwalda J G, Ross G J S, Stribley D P and Tinker P B 1982 The development of endomycorrhizal root systems IV. The mathematical analysis of effects of phosphorus on the spread of vesicular-arbuscular mycorrhizal infection in root systems. New Phytol, 92, 391–399.
Carling D E and Brown M F 1980 Relative effects of vesicular arbuscular mycorrhizal fungus on the growth and yield of soybeans. Soil Sci. Soc. of Am. J. 44, 528–532.
Carling D E and Brown M F 1982 Anatomy and physiology of vesicular-arbuscular and non-mycorrhizal roots. Phytopathol. 72, 1108–1114.
Clark R B 1983 Plant genotype differences in the uptake, translocation, accumulation and use of mineral elements required for plant growth. Plant and Soil 72, 175–196.
Clark R B, Maranville J W and Gorz H J 1978 Phosphorus efficiency of sorghum grown in limited phosphorus.In Proceedings of the 8th International Colloquim on Plant Analysis and Fertilizer Problems. Eds. A R Ferguson, R L Bieleski and I B Ferguson. pp93–99, Auckland, New Zealand.
Gianinazzi-Pearson V and Gianinazzi S 1983 The physiology of vesicular-arbuscular mycorrhizal roots. Plant and Soil 71, 197–209.
Giovanneti M and Mosse B 1980 An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 84, 489–500.
Hayman D S 1983 The physiology of vesicular-arbuscular endomycorrhizal symbiosis. Can. J. Bot. 61, 944–963.
Huismann O C 1982 Interrelations of root growth dynamics to epidemiology of root invading fungi. Ann. Rev. Phytopathol. 20, 303–327.
Jackson M L 1971 Son Chemical Analysis. pp 574. Prentice Hall of India (Ltd). New Delhi.
Menge J A 1983 Utilization of vesicular arbuscular mycorrhizal fungi in agriculture. Can. J. Bot. 61, 1015–1024.
Menge J A, Johnson E L V and Platt R G 1978 Mycorrhizal dependencey of several citrus cultivars under three nutrient regimes. New Phytol. 81, 553–559.
Menge J A, Steirle O, Bagyaraj D J, Johnson E L V and Leonard R T 1978 Phosphorus concentrations in plants responsible for inhibition of mycorrhizal infection. New Phytol 80, 575–578.
Mosse B 1980 Vesicular-arbusular mycorrhiza research for Tropical Agriculture. Research Bulletin, Hawaii Institute of Tropical Agriculture and Human Resources 194, 14–15.
Phillips J M and Hayman D S 1970 Improved procedures for clearing and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 55, 158–160.
Powell C L and Sithamparanathan J 1977 Mycorrhizas in Hill Country soils. IV. Infection rate in grass and legumes species by indigenous mycorrhizal fungi field conditions. N.Z.J. Agric. Res. 20, 489–502.
Smith S E and Walker N A 1981 A quantitative study of the mycorrhizal infection in Trifolium; separate determination of the rates of inefection and of mycelial growth. New Phytol. 89, 225–240.
Author information
Authors and Affiliations
Additional information
Journal Article No. 453
Rights and permissions
About this article
Cite this article
Krishna, K.R., Shetty, K.G., Dart, P.J. et al. Genotype dependent variation in mycorrhizal colonization and response to inoculation of pearl millet. Plant Soil 86, 113–125 (1985). https://doi.org/10.1007/BF02185031
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02185031