Abstract
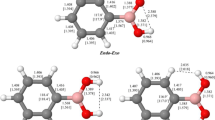
The most stable structure of CB2H3 −, as established computationally, is the aromatic diboracyclopropenyl (diboriranyl) anion (5), while open-chainC 2v, isomer H2BCBH (7) is only 3 kcal/mol higher in energy at the QCISD(T)/6-311 +G**//MP2/6-31+G*+ZPE (HF/6-31 +G*). The 47-kcal/mol barrier between cyclic,5, and open-chain,7, structures suggests that both of them may be observed. The aromatic stabilization energy of the diboriranyl anion (18 kcal/mol) is half the value in the isoelectronic cyclopropenium ion, C3H3 +. The computed, by IGLO method (5a), and experimental (6a) chemical shifts,δ(13C) andδ(11B), agree within 4 ppm range. The theoretical vibrational frequencies of the most stable isomers,5 and7, are presented for experimental verification of these species.
Similar content being viewed by others
References
Budzelaar, P. H. M.; Schleyer, P. v. R.J. Am. Chem. Soc. 1986,108, 3967;
Korkin, A.; Glukhovtsev, M.; Schleyer, P. v. R.Int. J. Quantum. Chem. 1993,46, 37.
Sundaralingam, A. M.; Lensen, L. H.J. Am. Chem. Soc. 1966,88, 198;
Breslow, R.Pure Appl. Chem. 1971,28, 111;
Ku A. T.; Sundaralingam, A. M.J. Am. Chem. Soc. 1972,94, 1688;
Krishnan, R.; Whiteside, R. A.; Pople, J. A.; Schleyer, P. v. R.J. Am. Chem. Soc. 1981,103, 5649;
Allen, F. H.Tetrahedron 1982,38, 645;
Lias, S. G.; Bartmess, J. E.; Liebman, J. F.; Holmes, J. L.; Levin, R. D.: Mallard, W. G.J. Phys. Chem. Ref. Data, Suppl. No. 1,1988,17, 109;
Wong, M. W.; Radom, L.J. Am. Chem. Soc. 1989,111, 6976;
Lammertsma, K.; Grüner, O. F.; Thibodeaux, A. F.; Schleyer, P. v. R.J. Am. Chem. Soc. 1989,111, 8995;
Lammertsma, K.; Schleyer, P. v. R.J. Am. Chem. Soc. 1990,112, 7955;
Li, W.-K.; Riggs, N. V.J. Mol. Struct. THEOCHEM,1992,89, 189.
Volpin, M. E.; Koreshkov, Y. D.; Dulova, V. G.; Kirsanov, D. N.Tetrahedron 1962,18, 107;
Krogh-Jespersen, K.; Cremer, D.; Dill, S. D.; Pople, J. A., Schleyer, P. v. R.J. Am. Chem. Soc. 1981,103, 2589;
Vander Kerk, S. M.; Budzelaar, P. H. M.; van der Kerk vom Hoof, A.; van der Kerk, G. J. M.; Schleyer, P. v. R.Angew. Chem. Int. Ed. Engl. 1983,22, 48
Budzelaar, P. H. M.; Kos, A. J.; Clark, T.; Schleyer, P. v. R.Organometallics 1985,4, 429;
Budzelaar, P. H. M.; Kraka, E.; Cremer, D.; Schleyer, P. v. R.J. Am. Chem. Soc. 1986,108, 561;
Eish, J. J.; Shafii, B.; Odom, J. D.; Rheinghold, A. L.J. Am. Chem. Soc. 1990,112, 1847;
Byun, Y.-G.; Saebo, S.; Pittman, C. U.J. Am. Chem. Soc. 1991,113, 3689.
Liang, C.; Allen, L. C.J. Am. Chem. Soc. 1991,113, 1878;
Paetzold, P.; Geret-Baumgarten, L.; Boese, R.Angew. Chem. Int. Ed. Engl. 1992,31, 1040;
Bühl, M.; Schaefer III, H. F.; Schleyer, P. v. R.; Boese, R.Angew. Chem. Int. Ed. Engl. 1993,32, 1154.
Brown, C. L.; Gross, K. P.; Onak, T.J. Am. Chem. Soc. 1972,94, 8055.
Wehrmann, R.; Meyer, H.; Bemdt, A.Angew. Chem. Int. Ed. Engl. 1985,24, 788.
Meyer, H.; Schmidt-Lukash, G.; Baum, G.; Massa, W.; Bemdt, A.Z. Naturforsch. 1988,43b, 801.
Grützmacher, H.Angew. Chem. Int. Ed. Engl. 1992,31, 1329;
Willershausen, P.; Schmidt-Lukash, G.; Kybart, C.; Allwohn, J.; Massa, W.; McKee, M. L.; Schleyer, P. v. R.Angew. Chem. Int. Ed. Engl. 1992,31, 1384;
Berndt, A.Angew. Chem. 1993,32, 985;
Korkin, A. A.; McKee, M. L.; Schleyer, P. v. R.Inorg. Chem. 1995,34, 961.
Gaussian 92, Revision E.3, Frisch, M. J.; Trucks, G. W.; Head-Gordon, M.: Gill. P. M. W.; Wong. M. W.; Foresman, J. B.; Johnson, B. J.; Schlegel, H. B.; Robb, M. A.; Replogle, E. S.; Gomperts, R.; Andres, J. L.; Raghavachari, K.; Binkley, J. S.; Gonzalez, C.; Martin, R. L.; Fox, D. J.; Defrees, D. J.; Baker, J.; Stewart, J. J. P.; Pople, J. A. Gaussian, Inc., Pittsburgh, PA, 1992.
Zero point energies at HF/6-31 +G* are scaled by 0.89 as recommended: Hehre. W. R.; Radom, L.; Schleyer, P. v. R.; Pople, J. A.Ab Initio Molecular Orbital Theory; Wiley: New York, 1986.
Dewar, M. J. S.The Molecular Orbital Theory of Organic Chemistry; McGraw-Hill: New York, 1969.
Krogh-Jesperson, K.; Cremer, D.; Poppinger, D.; Pople, J. A.; Schleyer, P. v. R.; Chandrasekhar, J.J. Am. Chem. Soc. 1979,101, 4843;
Jemmis, E. D.; Subramanian, G.; Naga Srinivas. G.J. Am. Chem. Soc. 1992,114. 7939.
Jug, K.J. Org. Chem. 1984,49, 4475;
Garratt, P. J.Aromaticity. Wiley: New York, 1986;
Minkin, V.; Glukhovtsev, M.; Simkin, B.Aromaticity and Antiaromaticity. Electronic and Structural Aspects. Wiley-Interscience: New York, 1994.
Alberts, I. L.; Schaefer III, H. F.Chem. Phys. Lett. 1990,165, 250.
Kutzelnigg, W.Isr. J. Chem. 1980,76, 1919;
Schindler, M.; Kutzelnigg, W.J. Chem. Phys. 1982,76, 1919;
For a review of IGLO applications, see Kutzelnigg, W.; Schindler, M.; Fleischer, U.In:NMR Basis Principles and Progress. Springer-Verlag: Berlin, 1990, p 165.
Huzinaga, S.Approximate Wave Functions; University of Alberta: Edmonton, Alberta, 1971.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Korkin, A.A., Schleyer, P.v.R., v. Arx, U. et al. The aromatic diboracyclopropenyl (diboriranyl) anion; CB2H3 −: An ab initio study. Struct Chem 6, 225–228 (1995). https://doi.org/10.1007/BF02293115
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02293115