Abstract
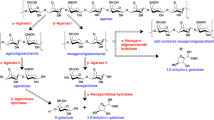
Cytophaga flevensis produced an inducible agarase which was extracellular under most conditions tested. The effect of cultural conditions on the production of enzyme was studied in batch and continuous culture. In batch culture, production was optimal whenCytophaga flevensis was incubated at 20C in a mineral medium with agar as the sole carbon source and ammonium nitrate as the nitrogen source at an initial pH of 6.6–7.0. The enzyme appeared to be subject to catabolite repression, since its synthesis was repressed when glucose was added to the medium in batch culture. Furthermore, in continuous culture, enzyme production decreased with increasing growth rate. Extracellular agarase was partially purified and the enzyme preparation obtained was very stable. The enzyme has a molecular weight of 26000 daltons. It is a β-agarase which is highly specific for polysaccharides containing neoagarobiose units. The final products of hydrolysis of agarose by the endo-acting enzyme were neoagarotetraose and neoagarobiose. Optimal conditions for its activity were pH 6.3 and 30C. When agarose was used as a substrate, an apparent temperature optimum of 35C was found, due to gelling of the substrate during the assay procedure.
Similar content being viewed by others
References
Araki, C. andArai, K. 1954. Studies on agar-digesting bacteria. The isolation of agardigesting bacteria and their enzymatic activities.—Mem. Fac. Ind. Arts, Kyoto Tech. Univ. (Sci. and Tech.)3B: 7–23.
Araki, C. andArai, K. 1956. Studies on the chemical constitution of agar-agar. XVIII. Isolation of a new crystalline disaccharide by enzymatic hydrolysis of agar-agar.—Bull. Chem. Soc. Japan29: 339–345.
Arnott, S., Fulmer, A., Scott, W. E., Dea, I. C. M., Moorhouse, R. andRees, D. A. 1974. The agarose double helix and its function in agarose gel structure.—J. Mol. Biol.90: 269–284.
Christison, J. andMartin, S. M. 1971. Isolation and preliminary characterization of an extracellular protease ofCytophaga sp.—Can. J. Microbiol.17: 1207–1216.
Davies, R. 1963. Microbial extracellular enzymes, their uses and some factors affecting their formation, p. 68–149.In C. Rainbow and A. H. Rose, (eds.), Biochemistry of industrial micro-organisms.—Academic Press, London and New York.
De Laey, M. etMassart, L. 1956. Hydrolyse enzymatique de l'agar.—Arch. Intern. Physiol. Biochim.64: 127–128.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A. andSmith, F. 1956. Colorimetric method for determination of sugars and related substances.—Anal. Chem.28: 350–356.
Duckworth, M. andTurvey, J. R. 1969a. An extracellular agarase from aCytophaga species. —Biochem. J.113: 139–142.
Duckworth, M. andTurvey, J. R. 1969b. The action of a bacterial agarase on agarose, porphyran and alkali-treated porphyran.—Biochem. J.113: 687–692.
Duckworth, M. andYaphe, W. 1970. Thin-layer chromatographic analysis of enzymic hydrolysates of agar.—J. Chromatogr.49: 482–487.
Duckworth, M. andYaphe, W. 1971. The structure of agar. Part II. The use of a bacterial agarase to elucidate structural features of the charged polysaccharides in agar.—Carbohyd. Res.16: 435–445.
Ishimatsu, K. 1952. Studies on the agar-liquefying bacteria (II). Influence of the culture conditions on the agarase production.—J. Ferm. Technol.30: 472–481.
Ishimatsu, K. 1953. Studies on the agar-liquefying bacteria (IV). Influence of temperature on the activity of agarase.—J. Ferm. Technol.31: 53–56.
Jobe, A. andBourgeois, S. 1973. Lac repressor-operator interaction. VIII. Lactose is an anti-inducer of thelac operon.—J. Mol. Biol.75: 303–313.
Kadota, H. 1953. Studies on the biochemical activities of marine bacteria. II. On the properties of agar-digesting enzyme ofVibrio purpureus.—Mem. Coll. Agr., Kyoto Univ.66: 31–38.
Lowry, O. H., Rosebrough, N. J., Farr, A. L. andRandall, R. J. 1951. Protein measurement with the Folin phenol reagent.—J. Biol. Chem.193: 265–275.
van der Meulen, H. J., Harder, W. andVeldkamp, H. 1974. Isolation and characterization ofCytophaga flevensis sp. nov., a new agarolytic flexibacterium.—Antonie van Leeuwenhoek40: 329–346.
Pollock, M. R. 1962. Exoenzymes, p. 121–178.In I. C. Gunsalus and R. Y. Stanier, (eds.), The Bacteria, Vol. IV.—Academic Press, New York and London.
Reese, E. T. andMaguire, A. 1969. Surfactants as stimulants of enzyme production by microorganisms.—Appl. Microbiol.17: 242–245.
Reese, E. T., Lola, J. E. andParrish, F. W. 1969. Modified substrates and modified products as inducers of carbohydrases.—J. Bacteriol.100: 1151–1154.
Robyt, J. F. andFrench, D. 1967. Multiple attack hypothesis of α-amylase action: action of porcine pancreatic, human salivary, andAspergillus oryzae α-amylases.—Arch. Biochem. Biophys.122: 8–16.
Sampietro, A. R. andVattuone de Sampietro, M. A. 1971. Characterization of the agarolytic system ofAgarbacterium pastinator.—Biochim. Biophys. Acta244: 65–76.
Somogyi, M. 1952. Notes on sugar determination.—J. Biol. Chem.195: 19–23.
Stahl, E. 1969. Thin-layer chromatography.—Springer-Verlag, Berlin, Heidelberg.
Stanier, R. Y. 1942. The Cytophaga group: a contribution to the biology of myxobacteria —Bacteriol. Rev.6: 143–196.
Swartz, M. N. andGordon, N. 1959. Agarase from an agar-digesting bacterium.—J. Bacteriol.77: 403–409.
Torriani, A. andPappenheimer, A. M., Jr. 1962. Inducible polysaccharide depolymerases ofBacillus palustris.—J. Biol. Chem.237: 3–13.
Turvey, J. R. andChristison, J. 1967a. The hydrolysis of algal galactans by enzymes from aCytophaga species.—Biochem. J.105: 311–316.
Turvey, J. R. andChristison, J. 1967b. The enzymic degradation of porphyran.—Biochem. J.105: 317–321.
Welker, N. E. andCampbell, L. L. 1963. Introduction of α-amylase ofBacillus stearothermophilus by maltodextrins.—J. Bacteriol.86: 687–691.
Yaphe, W. 1957. The use of agarase fromPseudomonas atlantica in the identification of agar in marine algae (Rhodophyceae).—Can. J. Microbiol.3: 987–993.
Young, K., Hong, K. C., Duckworth, M. andYaphe, W. 1971. Enzymic hydrolysis of agar and properties of bacterial agarases, p. 469–472.In Proc. 7th Int. Seaweed Symp.— University of Tokyo Press.
Young, K. S. 1972. An enzymatic and chemical study of agar.—Ph. D. Thesis, McGill University, Montreal, Canada.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van der Meulen, H.J., Harder, W. Production and characterization of the agarase ofCytophaga flevensis . Antonie van Leeuwenhoek 41, 431–447 (1975). https://doi.org/10.1007/BF02565087
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02565087