Abstract
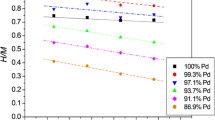
Linear expansion of thin-wall tubes of palladium-silver alloys containing 10, 20, 27, and 40 wt% silver was measured on absorption of small amounts of hydrogen (hydrogen-to-metal atom ratio, 0.005 to 0.25) at temperatures between 150°C (423 K) and 350°C (623 K) for equilibration with gaseous hydrogen at pressures up to around 1 bar. Hydrogen uptake by the test specimens was determined concurrently with the expansion measurements. Thermal expansion coefficients for the hydrogen-free materials were also obtained. Analysis of the data indicates that fractional length change is linearly dependent on hydrogen-to-metal atom ratio within the experimental range of hydrogen concentrations, independent of temperature, and varies only slightly with alloy composition. Partial molar volumes of hydrogen (with 95% confidence limits) vary from 1.77±0.08cm3·(mol H)−1 for the 10% Ag alloy to 1.92±0.03cm3·(mol H)−1 for the 40% Ag alloy. Hydrogen solubility results are correlated by a relation that facilitates linear extrapolation for determination of limiting values for equilibrium constants. Heats of solution of hydrogen at infinite dilution and standard entropy changes are essentially independent of temperature within the range of experimental conditions.
Similar content being viewed by others
References
T. Ooi, I. Ohno, and H. Numata,Denki Kagaku oyobi Kogyo Butsuri Kagaku 61:324 (1993).
E. S. Kodes, A. B. Zakharov, P. V. Gel’d, and N. I. Timofeev,Izv. Vyssh. Uchebn. Zaved. Fiz. 32:121 (1989).
R. Feenstra, R. Griessen, and D.G. DeGroot,J. Phys. F. Met. Phys. 16:1933 (1986).
E. A. Owen and J. I. Jones,Proc. Phys. Soc. (London) 49:587 (1937);49:603 (1937).
J. E. Worsham, Jr., M. K. Wilkinson, and C. G. Shull,J. Phys. Chem. Solids 3:303 (1957).
G. Nelin,Phys. Status Solidi 45:527 (1971).
N. F. Mott and H. Jones,The Theory and Properties of Metals and Alloys (Dover, New York, 1958), pp. 199–200.
C. E. Buckley, J. F. Dobson, and M. A. Poyser,J. Phys.: Condens. Matter 7: 5815 (1995)
R. Burch,Trans. Faraday Soc. 66:749 (1970).
Author information
Authors and Affiliations
Additional information
Paper dedicated to Professor Edward A. Mason.
Rights and permissions
About this article
Cite this article
Lindsay, W.T. Measurement of the partial molar volume and solubility of hydrogen in its dilute solutions in palladium-silver alloys. Int J Thermophys 18, 1051–1061 (1997). https://doi.org/10.1007/BF02575248
Issue Date:
DOI: https://doi.org/10.1007/BF02575248