Abstract
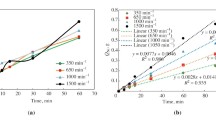
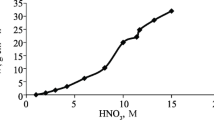
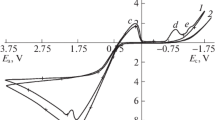
This article describes a kinetic study of the dissolution of silver and silver-gold alloys in nitric acid. For pure Ag, the nitric acid reaction order is two at low concentrations and one for concentrations above 4 M. When attacking alloys, the reaction order is one and drops to zero when the alloy contains 51.1 pct of silver by weight or less and the nitric acid concentration is 6 M or above. The activation energy is 12.1 kcal/mol in both cases. When the molar fraction of silver is 0.70 or more, the rate of silver dissolution from its gold alloys is controlled by the chemical reaction on the solid surface. When the molar fraction of the silver is less than 0.65 and nitric acid activity and temperature are high, the dissolution is controlled by the outward diffusion of silver nitrate through the undissolved gold layer. The dissolution rate is affected by the composition of the alloy. When the molar fraction of silver is 0.76 or more, the gold atoms are found in the lattice isolated or in pairs and silver atoms may dissolve without difficulty. Between 0.55 and 0.76, the reaction rate decreases quickly when the gold content increases because the atoms make up chains into lattice and this makes the dissolution difficult. When the molar fraction is less than 0.54, the alloy does not dissolve since the gold atoms form continuous surfaces that impede the attack.
Similar content being viewed by others
References
A. Butts and Ch.D. Coxe:SILVER, Economics, Metallurgy, and Use, Van Nostrand Co. Inc., Princeton, NJ, 1967, pp. 221–26.
T.K. Rose and W.A.C. Newman:The Metallurgy of Gold, 7th ed., Met-Chem Research Inc., Boulder, CO, 1937, pp. 47 and 501–03.
J.C. Yannopoulus:The Extractive Metallurgy of Gold, Van Nostrand Reinhold, New York, NY, 1991, p. 138.
J.L. Bray:Metalurgia Extractiva de los Metales no Ferreos, 2nd ed., Osca S.A., Madrid, 1968, p. 421.
D. Proske, H. Blumental, and F. Ensslin:Análisis de Metales, Métodos de Control Industrial, Aguilar, Madrid, 1960, vol. II, p. 641.
F. Habashi:Principles of Extractive Metallurgy, Gordon and Breach, New York, NY, 1980, vol. II, p. 40.
C.W. Ammen:Recovery and Refining of Precious Metals, Van Nostrand Reinhold, New York, NY, 1984, pp. 197 and 289.
E.M. Wise:Gold, Recovery, Properties and Applications, Van Nostrand Reinhold, New York, NY, 1964, p. 21.
H.P. Meissner and C.L. Kusic:AICHE Symp., 1978, Ser. 173, vol. 74, pp. 14–20.
H.P. Meissner:Am. Chem. Soc., 1980, pp. 495–511.
F. Habashi:Principles of Extractive Metallurgy, Gordon and Breach, New York, NY, 1980, vol. I, p. 127.
H.Y. Sohn and M.E. Wadsworth:Rate Processes of Extractive Metallurgy (Spanish Translation), Ed Trillas, Mexico DF, 1986, p. 305.
Author information
Authors and Affiliations
Additional information
Former Graduate Students, Department of Metallurgy, University of Barcelona.
Rights and permissions
About this article
Cite this article
Martínez, L.L., Segarra, M., Fernández, M. et al. Kinetics of the dissolution of pure silver and silver-gold alloys in nitric acid solution. Metall Trans B 24, 827–837 (1993). https://doi.org/10.1007/BF02663143
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02663143