Abstract
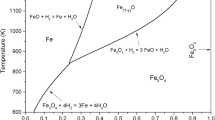
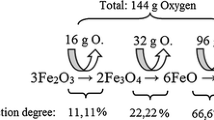
Many economic studies have shown that iron ore reduction could be an important use of hydrogen, provided that its price was low enough. With this in mind, it was important to clarify the reasons of the significant slowing down, observed between 700 °C and 900 °C, in many experimental investigations of iron ore (or oxide) reductions with hydrogen and attributed to very different reasons. The experiments reported here have been designed with wustite single crystals so that safer conclusions may be derived than with the more complex situation encountered with iron ores. TGA, coupled with microstructure investigations, has shown conclusively that the slowing down is connected with the iron texture evolution toward a layer more impermeable to diffusion in the pores. Such a behavior is still increased in wet hydrogen by the blocking of active sites on wustite by water vapor. Another result is worth stressing: the very irregular shape of the reaction interface, quite different from the classical topochemical model.
Similar content being viewed by others
References
R. Stephenson:Direct Reduced Iron, Publ. Iron and Steel Soc. AIME, Warrendale, PA, R.L. Stephenson, ed., 1980, pp. 160–84.
R. Quintero and T. Maeda:Proceedings of the Third World Hydrogen Energy Conference, Tokyo, T. N. Veziroglu, K. Fueki, and T. Otha, eds., June 1980.
K. Lilius:Acta Polytech. Scand., Chem. Met., 1974, Series no. 119, pp. 6-21.
M. Ray and L.De Jonghe:J. Mater. Sci., 1980, vol. 15, pp. 2241–52.
M. Tikkanen:Valtion Tek, Tutkimuslaitos, Julkaisu, 1949, no. 12, 92 pp.
A. Wilhelm and G. Saint Pierre:Trans. TMS-AIME, 1961, vol. 221, pp. 1267–69.
N. Themelis and W. Gauvin:A.I. Ch. E. Journal, 1962, vol. 8, pp. 437–44.
H. Lien, A. El Mehairy, and H. Ross:J. Iron Steel Inst., 1971, vol. 209, pp. 541–45.
E. Turkdogan and J. Vinters:Metall. Trans., 1971, vol. 2, pp. 3175–88.
K. Yokogawa and H. Iwai:Tekko Daigakushi, 1977, vol. 11, pp. 1–15.
Guillemonat, French Patent 1084764, 1953.
J. Henderson:Met. Soc. Conf., 1961, vol. 8, pp. 671–88.
H. Ulrich and K. Bohnenkamp:Arch. Eisenhut., 1965, vol. 36, pp. 611–18.
P. Strangway and H. Ross:Trans. TMS-AIME, 1968, vol. 242, pp. 1981–88.
B. Seth: Ph.D. Thesis, Univ. of Toronto, 1964.
I. Gaballah: Thesis, Univ. of Nancy, 1976.
M. Kochnev and A. Plotnikova:Izv. Akad. Nauk SSSR, Otd. Teck. Nauk, 1958, vol. 4, pp. 118–21.
I. Shohachi:Kagaku, 1958, vol. 28, pp. 34–41.
T. Rushton and S. Khalafalla: Bureau of Mines, U. S. Dept. of Int. Report 7080, 1968.
J. Quets, M. Wadsworth, and J. Lewis:Trans. TMS-AIME, 1960, vol. 218, pp. 545–50.
J. Szekely and J.W. Evans:Chem. Eng. Sci., 1971, vol. 26, pp. 1901–13.
E. Turkdogan and J. Vinters:Metall. Trans., 1972, vol. 3, pp. 1561–74.
M. McKewan:5th Int. Symp. Reactivity Solids, Munich, 1964, G. Schwab, ed., Elsevier, 1965, p. 623.
P. Hayes:Metall. Trans. B, 1979, vol. 10B, pp. 211–17.
M. Sastri, R. Viswanath, and B. Viswanathan:Trans. Indian Inst. Met., 1979, vol. 32, pp. 313–17.
P. Hayes and P. Grieveson:Metall. Trans. B, 1981, vol. 12B, pp. 579–87.
S. Taniguchi, M. Ohmi, and H. Hukuhara:Trans.ISIJ, 1980, vol. 20, pp. 753–58.
O. Gellner and F. Richardson:Nature, 1951, vol. 168, pp. 23–24.
K. Lilius:Acta Polyteck. Scand. Chem. Met. Series, 1974, no. 118, pp. 6-18.
S. El Moujahid: Ecole Centrale, Chatenay Malabry, unpublished research, 1981.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moukassi, M., Steinmetz, P., Dupre, B. et al. A study of the mechanism of reduction with hydrogen of pure wustite single crystals. Metall Trans B 14, 125–132 (1983). https://doi.org/10.1007/BF02670879
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02670879